Introduction
The methyl red test is a commonly used test in microbiology that is used to determine the ability of bacteria to perform mixed acid fermentation. Mixed acid fermentation is a metabolic process by which bacteria produce a mixture of different organic acids as end products of glucose metabolism. The methyl red test is based on the ability of certain bacteria to produce large amounts of acidic end products, which can then be detected using an indicator dye called methyl red. This test was first described by Voges and Proskauer in 1898, and it has since become an important tool for identifying and characterizing bacterial species.
The methyl red test is also a part of the IMViC (Indole, Voges-Proskauer, and citrate) test series, which is a group of tests used to differentiate between different species of enteric bacteria based on their metabolic properties.
Purpose of methyl red test
The purpose of the methyl red test is to determine the ability of bacteria to perform mixed acid fermentation. This test is based on the principle that some bacteria are able to produce large amounts of acidic end products, such as lactic acid, acetic acid, formic acid, and succinic acid, as a result of glucose metabolism. These acidic end products can then be detected using an indicator dye called methyl red.
Principle of Methyl Red (MR) test
In the methyl red test, a culture of bacteria is grown in a medium containing glucose as the sole carbon source. If the bacteria are able to perform mixed acid fermentation, they will produce large amounts of acidic end products, which will lower the pH of the medium. When the pH of the medium drops below a certain threshold, the methyl red indicator dye will change color from yellow to red.
The color change of the methyl red indicator dye indicates a positive result for the test, meaning that the bacteria are able to perform mixed acid fermentation. On the other hand, if the bacteria are not able to perform mixed acid fermentation, they will produce only small amounts of acidic end products, which will not lower the pH of the medium enough to cause a color change in the methyl red indicator dye. This will result in a negative result for the test.

Bacterial Species That Are Tested Using the Methyl Red (MR) Test
| Bacterial Species | Methyl Red Test Result |
| Escherichia coli | Positive |
| Salmonella spp. | Negative |
| Shigella spp. | Negative |
| Proteus vulgaris | Positive |
| Klebsiella pneumoniae | Positive |
| Enterobacter aerogenes | Positive |
| Citrobacter freundii | Positive |
Materials Required for the Methyl Red (MR) Test
The following are the materials required for performing the methyl red test:
- Methyl red indicator solution: This pH indicator dye changes color from yellow to red in acidic conditions. It can be purchased commercially or prepared in the laboratory.
- Glucose broth: This is a liquid medium containing glucose as the sole carbon source. It can be purchased commercially or prepared in the laboratory.
- Test tubes with screw caps: These are sterile test tubes used to hold the bacterial culture and the medium.
- Inoculating loop or needle: This is a sterile instrument used to transfer the bacterial culture to the medium.
- Incubator: This is a device used to maintain a controlled temperature for the growth of bacteria. The incubation temperature for the methyl red test is typically around 35-37°C.
- Sterile pipettes or droppers: These are used to add the methyl red indicator solution to the test tubes.
- Distilled or deionized water: This is used to prepare the medium and dilute the indicator solution if necessary.
- Bacterial culture: This is a pure culture of the bacterial species to be tested. It can be obtained from a clinical or environmental sample or purchased commercially.
- Bunsen burner or alcohol lamp: This is used to sterilize the inoculating loop or needle before and after use.
Procedure of Methyl red (MR)Test
The procedure for performing the methyl red test is as follows:
- Prepare the glucose broth medium according to the manufacturer’s instructions or as per laboratory protocols. Sterilize the medium by autoclaving or by using a sterile filtration technique.
- Inoculate the glucose broth medium with a pure culture of the bacterial species to be tested using a sterile inoculating loop or needle. Incubate the tubes at around 35-37°C for 24-48 hours or until visible growth is observed.
- Prepare a stock solution of the methyl red indicator by dissolving 0.1 g of MR in 300 ml of 95% ethanol and add distilled or deionized water upto 500ml. Sterilize the solution by filtration using a 0.22 µm filter or by autoclaving.
- After incubation, add 2-3 drops of the sterile methyl red indicator solution to each of the test tubes containing the bacterial culture. Gently mix the contents of the tube to ensure even distribution of the indicator.
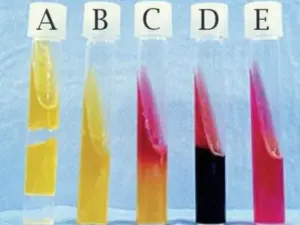
- Observe the color of the medium in the test tube. A positive result for the methyl red test is indicated by a red color, while a negative result is indicated by a yellow color.
If necessary, confirm the test result by comparing the color of the test tube with a control tube containing sterile glucose broth and the methyl red indicator.
Interpretation of the Methyl Red Test
Interpretation of the Methyl Red Test results involves the observation of the color change in the bacterial culture after the addition of the methyl red reagent. The methyl red reagent is a pH indicator that changes color from yellow to red in acidic conditions.
Positive Results
A positive result is indicated by the presence of a deep red color in the bacterial culture, indicating the production of acidic end-products during glucose fermentation. This result suggests that the bacteria belong to the group of organisms that produce mixed acid fermentation, which includes species such as Escherichia coli and Klebsiella pneumoniae.

Negative Results
A negative result is indicated by the absence of a color change or the presence of a yellow color in the bacterial culture. This result suggests that the bacteria do not produce acidic end-products during glucose fermentation and belong to the group of organisms that produce non-acidic end-products, such as Enterobacter aerogenes and Proteus vulgaris.
It is important to note that the Methyl Red Test should be used in conjunction with other biochemical tests to confirm the identification of bacterial species.
Advantages of Methyl red (MR) test
- Cost-effective: The test is relatively inexpensive and can be performed using basic laboratory equipment.
- Specificity: The test is highly specific for detecting acid production by certain bacterial species, which can aid in the differentiation of closely related bacterial strains.
- Sensitivity: The test is sensitive and can detect low levels of acid production, even in mixed cultures.
Limitations
- Limited scope: The test is limited to detecting acid production by only a few bacterial species, such as Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, and Enterobacter aerogenes.
- False-negative results: The test may give false-negative results if the bacterial culture is not in the exponential phase of growth, the medium is not properly prepared, or the test is not performed correctly.
- False-positive results: The test may give false-positive results if the medium is contaminated with other microorganisms that produce acidic end-products, or if the culture contains other strains of bacteria that also produce acidic end-products.
- Lack of quantification: The test only indicates whether the acid is produced or not, and does not provide information on the amount of acid produced. This limits its use for quantitative analysis.
- Interpretation: The interpretation of test results may be subjective, and slight variations in color or pH may affect the test outcome.
- Long incubation: It takes 48 hours for incubation to test the results
Comparison of Methyl Red (MR) test
Here is a comparison of the Methyl Red Test with other similar tests:
Voges-Proskauer Test: This test is used to detect the production of acetoin, a neutral end-product of glucose fermentation. The test is performed by adding the reagents alpha-naphthol and potassium hydroxide to the bacterial culture, followed by incubation. A positive result is indicated by the development of a red color. The Voges-Proskauer Test is complementary to the Methyl Red Test, as it detects organisms that produce neutral end-products, while the Methyl Red Test detects those that produce acidic end-products.
Citrate Utilization Test: This test determines whether a bacterium can utilize citrate as a carbon source. The test is performed by inoculating the bacterial culture onto a citrate agar medium and incubating it. A positive result is indicated by a change in color of the medium from green to blue, and sometimes by the development of growth. The Citrate Utilization Test is different from the Methyl Red Test in that it detects the ability of bacteria to utilize citrate, rather than the production of acidic end-products.
Clinical Applications of the Methyl Red Test
The Methyl Red Test is a biochemical test used to detect the production of acidic end-products by bacteria during glucose fermentation. This test has several clinical applications, including:
- Identification of bacterial pathogens: The Methyl Red Test can be used to identify certain bacterial species, such as Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, and Enterobacter aerogenes. These bacteria can cause a variety of clinical infections, such as urinary tract infections, sepsis, and pneumonia. Accurate identification of these pathogens is essential for appropriate antimicrobial therapy.
- Monitoring of bacterial growth: The Methyl Red Test can be used to monitor the growth of bacterial cultures in laboratory settings. The production of acidic end-products during glucose fermentation can indicate active bacterial growth, while a lack of acid production can indicate bacterial death or inhibition.
- Research purposes: The Methyl Red Test is used extensively in research to study bacterial metabolism and physiology. The test can provide information on the metabolic pathways and end-products of bacterial fermentation, which can aid in the understanding of bacterial pathogenesis.
Conclusion
In conclusion, the Methyl Red Test is a simple and rapid biochemical test used to detect the production of acidic end-products by bacteria during glucose fermentation. The test has several clinical applications, including identification of bacterial pathogens, monitoring of bacterial growth, and research purposes. The Methyl Red Test is a valuable tool in clinical microbiology for the characterization and identification of bacterial strains.
References
- https://asm.org/getattachment/0c828061-9d6f-4ae7-aea3-66e1a8624aa0/Methyl-Red-and-Voges-Proskauer-Test-Protocols.pdf
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA.
- Clinical Microbiology Procedures Handbook, Fourth Edition. (2016). In Clinical Microbiology Procedures Handbook, Fourth Edition. American Society of Microbiology. https://doi.org/10.1128/9781555818814
- Procop, G. W., Church, D. L., & Koneman, E. W. (2020). Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. Jones & Bartlett Learning.