Definition
Kligler’s Iron Agar (KIA) Test is a biochemical test used to examine bacteria’s ability to ferment glucose or lactose (or both) in a variety of ways; or lack of ability to ferment them at all. It is also used to test the ability of bacteria to generate H2S gas.
Introduction to Kligler’s iron agar test
To generate metabolic energy, bacteria use multiple enzyme systems and biochemical activities. The final product of metabolism of the same substrate may change between bacterial species depending on the enzyme system and biochemical properties. Some bacteria can ferment carbohydrates like glucose and lactose and produce acid, while others can only ferment one or none. Some of these sugars generate gas during fermentation, whereas others do not.
Kligler developed the Kligler’s lead acetate medium in 1918 to detect H2S generation and separation of Salmonella and Shigella species. To identify enteric bacilli, it was mixed with Russel’s Double Sugar Agar Medium. Bailey and Lacey then modified the mixed medium by substituting the Andrade indicator with phenol red and creating a new medium called Kligler’s Iron Agar medium, as well as developed the KIA test.
Importance of Kligler’s iron agar test
The KIA test uses KIA (Kligler’s Iron Agar) medium to distinguish bacteria based on their ability to generate H2S, gas, and acid by fermenting glucose, lactose, or both. This test primarily distinguishes Gram-negative enteric bacilli in clinical or non-clinical samples.
This test is similar to the TSI (Triple Sugar Iron Test), with the exception that the TSI agar in the TSI test contains a third sugar, sucrose, so it will not distinguish between lactose fermenter and non-lactose fermenter because the result will be the same if the bacteria ferment the sucrose or the lactose or both.
Objectives of Kligler’s iron agar test
- To distinguish Gram-negative enteric bacilli
- To assess the bacteria’s capacity to generate H2S, gas, and acid by fermenting glucose, lactose, or both.
- Lactose fermenters must be distinguished from non-lactose fermenters.
Principle of Kligler’s iron agar test
The ability of bacteria to use glucose, lactose, and sulphur compounds varies from bacteria to bacteria.
Glucose fermenters
Bacteria that can ferment glucose, generate metabolic acids. The acid formed during metabolism will lower the pH of the medium, turning it yellow in both the slant and butt areas. However, because the amount of glucose is less, it may exhaust quickly.
Lactose non-fermenters
If the bacteria are lactose non-fermenters, they are unable to utilize lactose as a carbon source, and the slant’s oxidative metabolism will be triggered by the lack of glucose. This oxidative metabolism raises the medium’s pH to an alkaline level and changes the yellow color of slant to red. However, because there is no or very little oxygen in the butt, oxidative degradation of peptone will not occur, and the butt will remain yellow. As a result, bacteria that do not digest lactose but do ferment glucose grow a red slant and a yellow butt.
Lactose fermenters
If the bacteria are lactose fermenters, they will ferment the lactose and produce acid. The released acid lowers the pH of the medium, and the indicator causes it to turn yellow in both the slant and butt areas. Because lactose is abundant, the oxidative metabolism of peptone is not activated. Thus, bacteria that ferment lactose (either lactose alone or lactose + glucose) generate a yellow slant and yellow butt.
Glucose and lactose non-fermenters
The medium will remain red if the bacteria are unable to ferment both sugars.
Gas production
The creation of gas bubbles above the medium or cracking/displacement of the medium will indicate gas production during sugar fermentation.
H2S production
H2S-producing bacteria can metabolize the sodium thiosulfate present in the culture media and release H2S gas. The generated H2S gas subsequently interacts with the ferric ions to form water-insoluble black ferrous sulphide. This insoluble black substance turns the culture media black, indicating H2S generation.
Requirements of Kligler’s iron agar test
- Culture Media Kligler’s Iron Agar (KIA) medium
- Bunsen burner
- Weighing Machine
- Inoculating loop
- Autoclave
- test tubes
- Incubator
- sample bacteria
Kligler’s Iron Agar Media Composition
| Components | Quantity grams/1L |
| Peptone | 15.0 |
| Beef Extract | 3.0 |
| Protease Peptone | 5.0 |
| Yeast Extract | 3.0 |
| Dextrose | 1.0 |
| Lactose | 10.0 |
| Sodium Chloride | 5.0 |
| Ferrous Sulfate | 0.20 |
| Glucose | 1.0 |
| Sodium Thiosulfate | 0.30 |
| Phenol Red | 0.024 |
| Agar | 15.0 |
| Final pH should be 7.4±0.2 at 25°C | |
Preparation of media
Must read: culture media preparation best ways to avoid contamination
- Measure the appropriate amount of media components as mentioned above and mix it with the needed volume of water (1 liter) in a glass beaker.
- Stir all of the components or boil if needed to dissolve all of the components completely in water.
- For 15 minutes, autoclave the tubes at 121°C and 15 lbs. pressure.
- Fill each test tube with roughly 5 to 7 mL of medium and loosely close the top or cloth plug into the opening.
- Allow it to cool and harden to make a slant (about 30° inclinations) to form an agar slant with a deep butt.
Protocol of Kligler’s iron agar test
- Touch a well-isolated colony of the test bacterium from a fresh culture (18 to 24 hours old) with a sterile inoculating wire.
- Inoculate the KIA medium tube by piercing the butt up to 3 to 5 mm above the tube’s base and streaking the slant part while withdrawing.
- For around 24 hours, incubate the tube aerobically at 37°C.
- Examine the slant and butt for color change and report the results within 24 hours of incubation. (If you need to examine the H2S production, incubate it for another 24 to 48 hours, but read the sugar fermentation and color change within the first 24 hours.)
Interpretation of results
- The red color of slant and yellow butt (Red/Yellow or Alkaline (K)/Acidic (A)) show a glucose fermenter and a lactose non-fermenter, respectively.
- Lactose fermentation (either lactose alone or combination lactose and glucose fermentation) is indicated by yellow slant and yellow butt (Yellow/Yellow or Acidic (A)/Acidic (A)).
- Lactose and glucose non-fermenters are indicated by red slant and red butt (Red/Red or Alkaline (K)/Alkaline (K)).
- H2S generation is indicated by blackening of the media or the appearance of black-colored zones.
- Gas production is indicated by cracking in the media, gas bubbles in the media, or the emergence of a breach in the media.
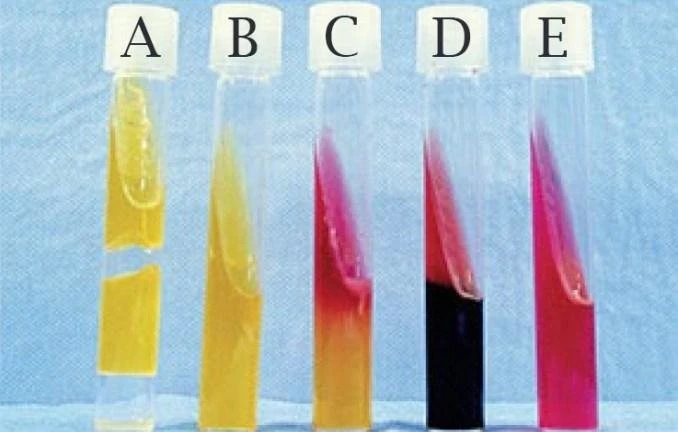
Figure 1 : this figure represents Tubes of Kligler’s iron agar (KIA) with a variety of reaction patterns A: acid/ acid, B: acid/acid with no gas production, C: Alkaline/Acid; D: Alkaline/Acid, H2S+; E: Alkaline/Alkaline (Ebob and Research 2020)
Expected results of KIA test
Carbohydrate Fermentation
- Positive result for Slant Reaction: Yellow (acid)
- Negative result for Slant Reaction: Red (alkaline)
- Positive result for Butt Reaction: Yellow (acid)
- Negative result for Butt Reaction: Red (alkaline)
KIA Color Reactions
- Red slant/ yellow butt: glucose (+), lactose (-)
- Yellow slant/ yellow butt: glucose (+), lactose (+)
- Red slant/ red butt: glucose (-), lactose (-)
Hydrogen Sulfide Production
- Positive Test is indicated by black color throughout medium, a black ring at the juncture of the slant and butt, or a black precipitate in the butt
- Negative Test is indicated no black color development
Gas Production:
- Positive Test is indicated by bubbles in the medium, cracking and displacement of the medium, or separation of the medium from the side and bottom of the tube
- Negative Test is indicated by No bubbles formation and no separation or displacement of the medium.
Reaction of some bacteria on KIA medium
| Bacteria | H2S production | Gas production | Slant color | Butt color | Slant pH | Butt pH |
| E. coli | -ve | + ve | Yellow | Yellow | Acidic | Acidic |
| Pseudomonas aeruginosa | -ve | -ve | Red | Red | Alkaline | Alkaline |
| K. pneumoniae | -ve | +ve | Yellow | Yellow | Acidic | Acidic |
| Enterobacter cloacae | -ve | +ve | Yellow | Yellow | Acidic | Acidic |
| K. oxytoca | -ve | + ve | Yellow | Yellow | Acidic | Acidic |
| Shigella spp. | -ve | -ve | Red | Yellow | Alkaline | Acidic |
| Proteus mirabilis | +ve | -ve | Red | Yellow | Alkaline | Acidic |
| Serratia marcescens | -ve | Variable | Red | Yellow | Alkaline | Acidic |
| Salmonella Typhi | -ve | + ve | Red | Yellow | Alkaline | Acidic |
| Yersinia enterocolitica | -ve | Variable | Red | Yellow | Alkaline | Acidic |
| Enterobacter cloacae | -ve | + ve | Yellow | Yellow | Acidic | Acidic |
| Salmonella Paratyphi B and C | + ve | +ve | Red | Yellow | Alkaline | Acidic |
| Providencia stuartii | -ve | -ve | Red | Yellow | Alkaline | Acidic |
| Morganella species | -ve | Variable | Red | Yellow | Alkaline | Acidic |
| Vibrio cholerae | -ve | -ve | Red | Yellow | variable | variable |
Applications of KIA test
- Gram-negative bacilli identification.
- To identify and distinguish members of the Enterobacteriaceae family.
- It is used to differentiate typhoid, dysentery, and other bacilli.
- Salmonella Typhi is distinguished from other Salmonellae, such as Salmonella Paratyphi A from Salmonella Scottmuelleri and Salmonella Enteritidis.
- Differentiating between lactose and non-lactose fermenters.
- H2S production detection can be done by this test.
- This test is used in fecal culture screening.
Disadvantages of KIA test
- It has a time limit, and it is recommended that the result be reviewed within 18 to 24 hours, not soon or too late.
- When inoculating Kligler Iron Agar, only pure cultures should be used. Observations may have errors if inoculated with a mixed culture.
- This test is invalid and null if the butt is not stabbed.
- Lactose fermentation alone and in combination with glucose produce the same result. As a result, it is not good for distinguishing based on lactose and glucose fermentation ability.
- Because H2S results in the medium black, it may be difficult to notice the color of the slant and butt.
- It is suggested that biochemical, molecular, immunological or mass spectrometry tests be undertaken on pure culture colonies for complete identification.
- Certain species or strains may exhibit delayed reactions or fail to ferment the carbohydrate in the specified time.
- Hydrogen sulphide examinations on Kligler Iron Agar is limited to Enterobacteriaceae members.
Precautions of KIA test
- If the medium has cracks or there is a gap in the medium before inoculation, do not use.
- When stabbing, do not use an inoculating loop since it may lead to cracking, which will cause confusion when reporting the gas generation.
- Prior to 18 hours of incubation, do not read the sugar utilization result since the glucose may not be reduced and the oxidative metabolism of peptones may not have begun.
- After 24 hours, do not read the results because the entire tube may turn black and fail to reflect the color change of the medium.
- Remove the tubes from the incubator and place them in a freezer at 4°C if you need to read the results after 24 hours.
Conclusion
Kligler’s Iron Agar (KIA) is a differential and selective medium used in microbiology to identify and differentiate enteric bacteria, especially those in the Enterobacteriaceae family. It enables microbiologists to analyze these bacteria’s ability to ferment certain carbohydrates (glucose and lactose) as well as their production of hydrogen sulphide gas. The results of KIA tests are useful for categorizing bacteria into distinct groups based on fermentation patterns and hydrogen sulphide generation. This test is used in clinical and research laboratories to aid in the diagnosis and characterization of bacterial species and is a vital tool in the preliminary identification of intestinal bacteria.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Downes F. P. and Ito K., (Ed.), 2001, Compendium of Methods for the Microbiological Examination of Foods, 4th Ed., American Public Health Association, Washington, D.C.
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier
- https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/KliglerIronAgarKIA.htm
- Kligler’s Iron Agar (KIA): Principle, Procedure, Results • Microbe Online
- Ebob TJJoAiM, Research M. 2020. A Review on Diagnostic Methods for the Identification of Vibrio cholerae. 32(8): 136-164.







