Introduction
The oxidative fermentative test determines whether bacteria can metabolize carbohydrates oxidatively, through fermentation, or are non-saccharolytic i.e. unable to utilize the carbohydrates in the media. Carbohydrates are organic compounds that contain (CH2O)n carbon, hydrogen, and oxygen. Carbohydrate is used differently by various organisms based on their enzyme complement. Because fermentation patterns are characteristics of certain species, genera, or groups of microorganisms, this trait has been widely used as a method for the biochemical differentiation of bacteria.
Only under aerobic conditions, oxidative microorganisms can metabolize glucose or other sugars, indicating that oxygen is the ultimate hydrogen acceptor. Other species break down glucose and use another molecules, such as sulphur, as a hydrogen acceptors. This fermentative process is oxygen-free, and organism cultures can be aerobic or anaerobic. An acid is the end product of carbohydrate metabolism.
What is difference between fermentation test and oxidative-fermentative test?
The essential principle that distinguishes fermentation testing from oxidative-fermentation tests is the metabolic pathways they follow. Fermentation tests are used to determine whether a microorganism can metabolize certain carbohydrates or sugars via fermentation. Microorganisms break carbohydrates in an anaerobic (oxygen-free) environment, producing end products such as acids and gases such as carbon dioxide. The presence of acid and/or gas generation in the test media determines if fermentation happened. Conversely, oxidative-fermentation (OF) tests adopt a broader approach. They analyze not only fermentation but also the microorganism’s ability to perform oxidative metabolism, which occurs in the presence of oxygen.
History of oxidative-fermentative test
- Fermentation was once characterised as the process by which acid was produced through the metabolism of carbohydrates in both aerobic and anaerobic metabolism.
- Hugh and Leifson described the oxidative-fermentative (OF) test in 1953. Fermentation was originally defined as the process by which acid formed through carbohydrate metabolism in both aerobic and anaerobic metabolism.
Hugh and Leifson developed the term “oxidative” to characterize the mechanism by which acid is formed from carbohydrates in the presence of oxygen.
Aerobic carbohydrate utilization by bacteria results in less acid generation than fermentative metabolism. Glycogen was the principal carbohydrate source in the first OF medium. They reduced the quantity of peptone and increased the amount of glucose in this medium compared to the medium used to detect fermentation. As a result, even during oxidative metabolism, the alkaline byproduct of peptone metabolism was reduced and acid production was elevated in this medium.
For the first time, scientists were able to distinguish gram-negative bacteria that oxidize glucose from those that ferment it in this medium. These characteristics subsequently proved vital for classifying gram-negative bacteria. Prior to Hugh and Liefson’s research, taxonomists paid little attention to the type of carbohydrate breakdown. This test is one of the most significant in the identification of aerobic bacteria, Cowan and Steele noted in their 1965 manual known as Identification of Medical Bacteria.
Objective of oxidative-fermentative test
This test aims to detect and examine bacteria that either they can do oxidation or fermentation of sugars or carbohydrates or not.
Principle of oxidative-fermentative test
Hugh and Leifson’s medium, also known as OF media, contains tryptone and bromothymol blue (an indicator) and can be used to evaluate if an organism is oxidative or fermentative. The medium contains one of the sugars, such as glucose, mannitol, xylose, sucrose, lactose, or maltose, which serves as the fermentable carbohydrate.
An organism is added to two tubes of OF Medium. After inoculation, one tube is coated with mineral oil or melted paraffin to form an anaerobic environment. The other tube remains open to the environment. Microorganisms grow in this medium by either using tryptone, which produces an alkaline reaction (dark blue color), or by using glucose, which produces acid (changing bromothymol blue to yellow).
The oxidative metabolism of the carbohydrate will result in acid formation (yellow) only in the open tube. Fermentative carbohydrate breakdown will result in acid production (yellow) in both the open and closed tubes. Acidic changes in the overlay tubes are thought to be the result of valid fermentation, whereas acidic changes in the open tubes are thought to be a result of oxidative metabolism of the carbohydrate present. Acid will not be produced by asaccharolytic microbes in either tube.
Requirements of oxidative fermentative test
Media
Hugh and Leifson’s medium or OF basal media
Reagents
- Sterile mineral oil OR
- Liquid paraffin OR
- Sterile melted petrolatum
Apparatus
- Bunsen burner
- Test tubes
- Inoculating wire
- Autoclave
- Incubator
Test organisms
- Test organisms ( Gram-negative bacilli) pure well-isolated colonies from an 18-24-hour culture
- Glucose Fermenter: Escherichia coli
- Glucose oxidizer: Pseudomonas aeruginosa
- Asaccharolytic: Alcaligenes faecalis
Media composition
| Components | Quantity grams/1L/ |
| Peptone | 2.0 |
| Sodium chloride | 5.0 |
| Dipotassium phosphate | 0.30 |
| Glucose (Dextrose) | 10.0 |
| Bromothymol blue | 0.030 |
| Agar | 3.0 |
| Final pH should be 7.1±0.2 at 25°C | |
Preparation of media
Take 1L distilled water in a glass beaker and mix the above given components. Prior to autoclaving, the pH should be adjusted to 7.1. After autoclaving the medium at 121°C for 15 minutes, a filter sterilized solution of 10% carbohydrate solution is aseptically added to the medium to a final concentration of 1%. The carbohydrate-containing sterile media is aseptically poured into sterile test tubes and cooled unslanted as stabs.
Prior to sterilization, some procedures require the addition of 10 g/liter of carbohydrate to the medium. Before pouring into test tubes, the medium is dissolved by bringing it to a boil on a hot plate or by steaming for 20 minutes. To prevent carbohydrate breakdown, the tubed medium is steamed for 20 minutes instead of autoclaving.
Protocol for oxidative-fermentative test
Method 1: for glucose test
- From an 18-24 hour culture, get pure, isolated colonies.
- Inoculate tubes in duplicate for each test organism. Inoculate the agar by stabbing it about 1/4 inch from the bottom.
- Pour one of each duplicate tube with sterile mineral oil, sterile melted paraffin, or sterile melted petrolatum. Tighten the overlay tube’s cap and loosen the non-overlaid tube’s cap.
- Incubate both tubes aerobically for up to 14 days at 35ºC.
- Check tubes for color changes on a regular schedule.
Method 2: for carbohydrates other than glucose
- Non-fermenting gram-negative rods that produce an oxidative result in an OF glucose test can be further investigated to discover if they can oxidatively metabolize more carbohydrates.
- In the medium, maltose, mannitol, lactose, or sucrose are used instead of glucose, and just one tube is utilized for each type of carbohydrate.
- Because many of these non-fermenters grow slowly, you should utilize a large amount of inoculum. There is no requirement for mineral oil or an agar layer because the test is based on aerobic respiration. The presence of acid and a change in pH at the top of the tube after 24 hours indicate that the test was successful.
- Some slow-growing non-fermenters may require several days to produce enough acid for the bromothymol blue to detect them.

Interpretation of results
Positive results
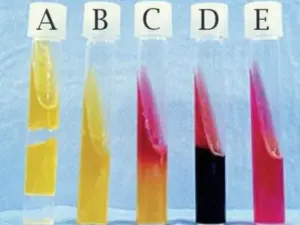
The appearance of a yellow color in the medium indicates a positive carbohydrate utilization test.
Oxidative
Non-fermenting bacteria that use oxidative metabolism to metabolize glucose provide an oxidative end result. The small amount of acid generation in the open tube indicates this result. The generated acid (pH 6.0) causes the pH indicator, bromothymol blue, to turn from green to yellow.
A change in pH is noticed at the surface of the open tube where growth in the presence of oxygen is observed after a 24-hour incubation. The less quantity of agar in the medium enables for eventual diffusion of the weak acid throughout the entire tube after long incubation (more than 48 hours). In the oil-covered tube, no color change or response occurs.
Fermentative
Bacteria that can ferment glucose produce acid in both the open (aerobic) and oil-covered (anaerobic) tubes, indicating a fermentative result. The generated acid (pH 6.0) causes the pH indicator, bromothymol blue, to turn from green to yellow. The medium’s semisolid nature also allows for the detection of motility. Take a look on the hazy growth distant from the stab line.
Negative results
Bacteria that are not saccharolytic produce a negative OF result. The absence of color change in the oil-covered tube and, in some situations, an increase in pH (pH 7.6) change the bromothymol blue color, from green to blue at the top of the open tube indicating a negative result. The pH rise is caused by bacteria producing amines, which break down the peptone (protein) in the medium. Other bacteria produce a negative outcome, as seen by no growth or color change in the media.
| Open test tube | Sealed test tube | Results |
| Yellow | Green | Oxidative bacteria |
| Yellow | Yellow | Fermentative bacteria |
| Green | Green | Cannot metabolize carbohydrate |
Important points to be considered
Instructions regarding medium of culture
- All identification tests should be performed using a non-selective medium. If the test is performed on selective agar, a purity plate must be provided to ensure the organism’s purity.
- Screw cap tubes should not be closed too tightly, to allow air exchange.
- Hugh and Leifson’s medium does not support the growth of many organisms. Repeat the test after enriching each tube with 2% serum or 0.1% yeast extract in this case.
Instructions regarding Incubation
Some organisms may require prolonged incubation before acid generation. This delayed reaction can be due to a carbohydrate’s difficulty in permeating the bacterial cell. So wait for the complete metabolism of carbohydrate
Instructions regarding Reaction
- The color change caused by oxidative organisms begins at the medium’s surface. It might not be obvious for several days. It is important not to misinterpret this as a negative reaction.
- Mineral oil should not be used since it is a heavy liquid petroleum that facilitates air diffusion.
- The OF test results should be classified as oxidative (O), fermentative (F), or negative (-). There is no indication of a good (+) outcome.
- Negative means that there is no fermentative or oxidative carbohydrate metabolism in the media.
Advantages of oxidative-fermentative test
- It helps to identify gram-negative bacteria based on their characteristics to oxidize or metabolize a specific carbohydrate.
- It is used to test whether an organism produces acid byproducts from a carbohydrate substrate.
- The ability of non-fermentative bacteria to generate acid from six carbs (glucose, sucrose, mannitol, lactose, xylose, and maltose) is commonly examined through this test.
Disadvantages of oxidative-fermentative test
- For complete identification of a microbe further testing is needed i.e. biochemical testing, immunological testing, molecular testing, and mass spectrometry testing on colonies from pure culture.
- Slow-growing organisms may take several days to give results.
- OF Medium does not support the growth of several bacteria. To confirm the negative reaction, an additional basal medium containing dextrose may be required.
- Some mineral oils are acidic, which might lead to incorrect results.
- Bacteria that solely oxidize dextrose are incapable of fermenting any other carbohydrate. Other carbs will be oxidized solely. As a result, when measuring other carbohydrate utilization of such organisms, the covered tube may be disregarded.
References
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA
- Murray, P.R., E.J. Baron, J.H. Jorgensen, M.L. Landry, and M.A. Pfaller. 2007. Manual of Clinical Microbiology. 9th ed. ASM Press, Washington, D.C.
- Parajuli NP, Bhetwal A, Ghimire S, Maharjan A, Shakya S, Satyal D, Pandit R, Khanal PRJIjogm. 2016. Bacteremia caused by a rare pathogen–Chromobacterium violaceum: a case report from Nepal. 441-446.
- Prasanna S, Dharanidevi S, Das NK, Raj SJIJCMAS. 2016. Prevalence, phenotypic characterization and antibiotic susceptibility of non-fermentative gram negative bacilli isolates at a Tertiary Care Centre. 5(11): 442-454.
- https://asm.org/ASM/media/Protocol-Images/Oxidative-Fermentative-Test-Protocol.pdf?ext=.pdf