Introduction
Citrate Utilization Test is a commonly used test in bacteriology to determine the ability of bacteria to use citrate as the sole carbon source for their growth. This test is an important tool for the identification and classification of bacteria based on their metabolic capabilities. Citrate Utilization Test is based on the ability of bacteria to produce the enzyme citrate lyase, which is responsible for the breakdown of citrate into pyruvate and carbon dioxide (CO2).
Importance of Citrate Utilization Test in Bacteriology
Citrate Utilization Test is important in bacteriology because it is a rapid and reliable method for the identification of certain bacteria, especially members of the Enterobacteriaceae family. This test is particularly useful in differentiating between species of the Enterobacteriaceae family, which are known to have varied metabolic capabilities. The Citrate Utilization Test is also useful in the identification of other bacteria that can utilize citrate, such as Pseudomonas aeruginosa.
Principle
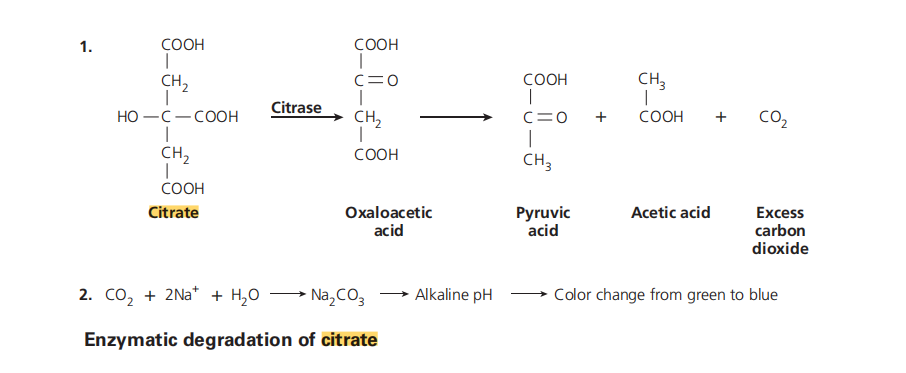
The principle behind Citrate Utilization Test is based on the ability of bacteria to utilize citrate as the sole carbon source for their growth. In the presence of citrate, the bacteria produce the enzyme citrase, which breaks down citrate into pyruvate and carbon dioxide (CO2). The production of CO2 increases the alkalinity of the medium and results in a change in color from green to blue, indicating a positive test result.
The role of citrate permease and citrase in citrate metabolism
Citrate permease is a transport protein located on the bacterial cell membrane that facilitates the transport of citrate from the extracellular environment into the bacterial cell. Once inside the cell, citrate is acted upon by citrase, an enzyme that is produced by bacteria capable of utilizing citrate. Citrase breaks down citrate into oxaloacetate and acetyl-CoA. Oxaloacetate is then converted to pyruvate, which can be used as a carbon source for the synthesis of various cellular components. CO2 is produced as a byproduct of the citrate metabolism pathway.
Conversion of citrate to pyruvate and CO2
The conversion of citrate to pyruvate and CO2 is a multistep process that involves several enzymatic reactions. The first step is the transport of citrate into the bacterial cell via citrate permease. Once inside the cell, citrate is converted to oxaloacetate and acetyl-CoA by the enzyme citrase. Oxaloacetate is then converted to pyruvate by the enzyme malate dehydrogenase. The conversion of oxaloacetate to pyruvate generates NADH, a reducing agent that can be used in other metabolic pathways. CO2 is produced as a byproduct of the reaction and is excreted from the cell.
Materials and Methods
List of required materials and equipment:
- Simmons Citrate Agar medium
- Inoculating loop
- Bacterial culture to be tested
- Incubator set to 37°C
- Sterile test tube
- pH indicator (Bromothymol blue)
Media
The most commonly used media for the Citrate Utilization Test is the Simmons Citrate Agar, which is a selective and differential medium. The medium contains ammonium dihydrogen phosphate, sodium chloride, magnesium sulfate, dipotassium phosphate, and bromthymol blue as the pH indicator. It also contains citrate as the sole carbon source. The agar is solidified using agar powder.
Reagents
The reagents used in the Citrate Utilization Test include Kovac’s Reagent and 0.5% ferric ammonium citrate solution.
Preparation of Media and Reagents:
Simmons Citrate Agar
a. Weigh out the required amount of Simmons Citrate Agar powder and add it to distilled water.
b. Heat the mixture to boiling and stir until the agar is completely dissolved.
c. Sterilize the medium by autoclaving at 121°C for 15 minutes.
d. After sterilization, allow the medium to cool to 45-50°C before use.
Kovac’s Reagent
a. Mix 5 mL of amyl alcohol and 0.2 g of p-dimethylaminobenzaldehyde in a test tube.
b. Add 15 mL of hydrochloric acid (37%) to the test tube and mix well.
c. Store the reagent in a dark bottle at room temperature.
0.5% Ferric Ammonium Citrate Solution:
a. Dissolve 0.5 g of ferric ammonium citrate in 100 mL of distilled water.
b. Sterilize the solution by autoclaving at 121°C for 15 minutes.
c. Allow the solution to cool to room temperature before use.
Note: It is important that all media and reagents should be prepared using sterile techniques to avoid contamination, and they should be stored properly to maintain their effectiveness.
Step-by-step procedure for performing Citrate Utilization Test
- Sterilize the inoculating loop by passing it through a flame until it turns red-hot.
- Dip the inoculating loop into the bacterial culture to be tested and pick up a small amount of the culture.
- Streak the inoculating loop onto the surface of the Simmons Citrate Agar medium, making sure to cover a small area of the surface.
- Incubate the plate in an incubator set to 37°C for 24-48 hours.
- Check the plate for growth and the change in color of the medium.
- Look for a change in color from green to blue, which indicates a positive test result.
Precautions to be taken during the test
- The inoculating loop must be sterilized before and after each use to prevent contamination of the bacterial culture or the medium.
- The bacterial culture used for the test must be a pure culture to avoid any cross-contamination.
- The Simmons Citrate Agar medium must be stored properly and used before its expiration date to ensure the accuracy of the test results.
- The plate should not be disturbed during incubation to prevent any interference with the growth of the bacteria.
- The incubation time and temperature should be strictly followed to ensure the optimal growth conditions for the bacterial culture.
- The pH indicator (Bromothymol blue) must be added to the medium as per the manufacturer’s instructions, and the color change should be interpreted accordingly.
- The test should be performed in a sterile environment to avoid contamination of the culture or the medium.
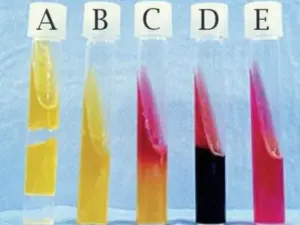
Interpretation of the results
Positive Results
A positive test result indicates that the bacterial culture can utilize citrate as the sole carbon source, which means that it produces the enzyme citrase that breaks down citrate into pyruvate and CO2. The production of CO2 increases the alkalinity of the medium, causing a color change from green to blue.
Negative results
A negative test result indicates that the bacterial culture cannot utilize citrate as a carbon source, which means that it does not produce the enzyme citrase required for the breakdown of citrate. The medium remains green as there is no increase in the alkalinity of the medium due to the absence of CO2 production.

Expected results for different types of bacteria
Different types of bacteria can have different results for the Citrate Utilization Test, depending on their metabolic capabilities. Here are some examples:
- Citrobacter freundii: Positive result, as it can utilize citrate as a carbon source.
- Escherichia coli: Negative result, as it cannot utilize citrate as a carbon source.
- Enterobacter aerogenes: Positive result, as it can utilize citrate as a carbon source.
- Klebsiella pneumoniae: Negative result, as it cannot utilize citrate as a carbon source.
- Salmonella enterica: Negative result, as it cannot utilize citrate as a carbon source.
- Citrobacter koseri: Positive result, as it can utilize citrate as a carbon source.
Limitations of Citrate utilization test
The Citrate Utilization Test, like any other diagnostic test, has its limitations. Some of the limitations of the test are:
- Slow-growing bacteria: Some bacteria grow slowly, and it may take longer than 48 hours for the test to show a positive or negative result.
- False negatives: Some bacteria may have the ability to utilize citrate, but they may not produce enough citrase to cause a color change in the medium. This may result in a false negative test result.
- False positives: Some bacteria may not be able to utilize citrate, but they may produce other enzymes that cause a color change in the medium, resulting in a false positive test result.
- Contamination: Contamination of the medium or the bacterial culture used for the test can lead to inaccurate results.
Possible sources of error
There are several possible sources of error in the Citrate Utilization Test, some of which are:
- Improper storage of the medium: If the medium is not stored properly, it may deteriorate, leading to inaccurate test results.
- Contamination: Contamination of the medium or the bacterial culture used for the test can lead to inaccurate results.
- Incomplete inoculation: Incomplete inoculation of the medium can lead to uneven growth, making it difficult to interpret the test results.
- Improper incubation conditions: If the incubation conditions are not optimal, it can affect the growth of the bacterial culture and the test results.
- Incorrect interpretation of results: Incorrect interpretation of the test results due to inadequate training or lack of experience can lead to inaccurate results.
Comparison with other tests for citrate metabolism
There are several tests available for the detection of citrate metabolism in bacteria, such as the Voges-Proskauer test, the Methyl Red test, and the Simmons Citrate Agar test. The Voges-Proskauer test and the Methyl Red test are biochemical tests that are used to detect the metabolic pathway of bacteria.
The Voges-Proskauer test is used to detect the production of acetoin, which is a by-product of glucose fermentation. The Methyl Red test is used to detect the production of mixed acids, which are produced during glucose fermentation.
References
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA.
- Clinical Microbiology Procedures Handbook, Fourth Edition. (2016). In Clinical Microbiology Procedures Handbook, Fourth Edition. American Society of Microbiology. https://doi.org/10.1128/9781555818814