Introduction
The latex agglutination test is a diagnostic procedure that involves particle clumping, or agglutination, in the presence of particular antibodies. It is a quick and dependable technique for detecting a wide range of analytes in clinical laboratories.
The test includes latex particles made of various organic materials that can be prepared to the appropriate diameter. These latex particles can also be functionalized with chemical groups to aid in molecule adhesion. Singer and Plotz presented the “Rheumatoid Factor Test” in 1956 as the first application of latex agglutination in a diagnostic test.
The latex agglutination test can be used to identify antigens as well as antibodies. It is a passive agglutination test in which either the antigen or the antibody is coated onto artificial carrier particles, also known as latex beads. Latex fixation tests are another name for these tests.
When a particulate or insoluble antigen is combined with its antibody in the presence of electrolytes at the proper temperature and pH, the particles clump or agglutinate. There are three types of agglutination reactions: direct, indirect (passive), and reverse passive.
The antigen agglutinates with the antibody directly in the direct agglutination test. The indirect or passive agglutination test, on the other hand, involves coating the antigen onto the surface of a carrier molecule, such as latex beads, and the antibody attaches to the coated antigen, causing agglutination on the surface of carrier molecule.
Principle of latex agglutination test
The binding of antibody or antigen molecules to the surface of latex beads is the basis of the latex agglutination principle. Because of the random alignment and attachment of antibody or antigen molecules, these latex beads, which are typically comprised of polystyrene, give a vast number of possible binding sites.
The latex beads are coated with antibodies in the case of antibody detection. Because each latex particle contains a large number of antibodies, there is a high density of exposed binding sites. Similarly, antigens are coated on latex beads in antigen detection. Numerous binding sites are provided by the huge number of antigen molecules on the surface of latex particles.
When a specimen is introduced to latex beads, the antigen or antibody present binds to the corresponding antigen or antibody exposed on the latex beads’ surface. As a result of this binding, cross-linked aggregates or clumps of latex beads and antigen or antibody will form.
The huge size of the latex particles aids in the visualization of the antigen-antibody response. The clumping or agglutination of latex beads can be seen with the naked eye or with the help of visual aids such as microscopy. The presence of visible clumps in the specimen shows a positive reaction, suggesting the presence of the relevant antigen or antibody.
Antibodies are adsorbed to latex particles under proper ionic and alkaline pH conditions in latex agglutination. The Fc portion of the antibodies is principally responsible for this binding, leaving the Fab region free to interact with the antigen present in the applied specimen.
Latex agglutination reagents’ sensitivity and lifespan have improved by using smaller latex particles. The smaller particle size allows for improved dispersion and interaction with the antigen or antibody, which improves the detection sensitivity of test. Furthermore, the increased reagent lifespan ensures that latex agglutination tests remain reliable and accurate over time.
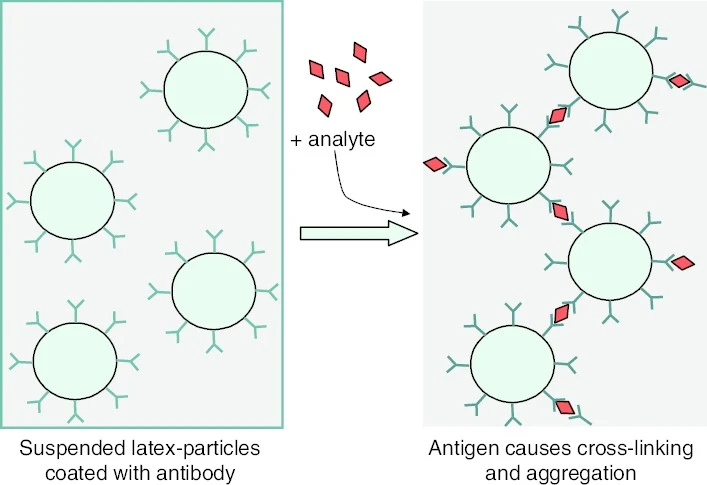
Figure 1: principle of latex agglutination test (Gubala et al. 2014)
Objectives of latex agglutination test
- One of the very important objectives of the latex agglutination test is to diagnose autoimmune disorders. The test can detect the presence of autoantibodies in a patient’s sample by coating latex beads with autoantigens associated with autoimmune diseases. The agglutination reaction between autoantibodies and coated autoantigens gives important information for autoimmune disease diagnosis.
- Microbial and viral illnesses are frequently detected with latex agglutination assays. The test can detect the presence of appropriate antibodies in the patient’s sample by coating latex beads with specific antigens related to the target pathogen. The presence of an infection is indicated by the agglutination reaction between antibodies and coated antigens, which aids in the diagnosis and management of infectious disorders.
- The latex agglutination test also seeks to detect analytes such as hormones, medications, or serum proteins. The test can detect the presence of these analytes in the patient’s sample by coating the latex beads with specific antigens or antibodies relevant to them. The analyte-coated particle agglutination reaction allows for quantitative or qualitative analysis of these compounds.
Types of latex agglutination test
Latex agglutination assays are classified into two types: antibody detection and antigen detection. The latex beads in the antibody detection test are coated with the specific antigen, whereas the latex beads in the antigen detection test are coated with the corresponding antibody.
Latex Agglutination Test (LAT) for Antibody Detection
This is referred to as a passive agglutination test. It includes coating the specific antigen on the surface of latex beads. This test is designed to detect the presence of antibodies in the test sample. When a sample, such as serum or other bodily fluids, is combined with antigen-coated latex beads, the antibodies in the sample bind to the antigen on the beads. This binding causes the latex beads to clump or agglutinate, which is a positive indicator of the existence of antibodies in the sample.
Antigen Detection Using the Latex Agglutination Test (LAT)
This test is known as a Reverse Passive Agglutination Test. The surface of latex beads gets coated with the particular antibody in this procedure. This test is designed to detect the presence of antigens in the test sample. When the sample is combined with antibody-coated latex beads, the antigens in the sample bind to the antibody on the beads. This binding causes the latex beads to clump or agglutinate, which is a positive indicator of the presence of antigens in the sample.
In distinct settings, these two forms of latex agglutination tests provide useful diagnostic information. The LAT for antibody detection is beneficial for analyzing the existence of specific antibodies in a patient’s sample, which can suggest a prior or current immunological response to a certain antigen. The LAT for antigen detection, on the other hand, is useful for detecting the presence of specific antigens, which may be symptomatic of an ongoing infection or the presence of foreign substances in the body.
Both methods of latex agglutination tests have advantages such as quick results, simplicity, and sensitivity. They have been widely utilised in clinical laboratories for the detection of numerous analytes, assisting in the diagnosis and management of infectious diseases and immune-related ailments.
Materials Required for Latex Agglutination Test
Apparatus
- 1.5 ml Vials
- Micro-centrifuge
- Glass slides
- Pipette
- Microtips
- Laboratory refrigerator
- Beakers
- Latex beads: these are primarily formed of polystyrene, serve as the carrier particles in the latex agglutination test. Depending on the type of test, they are coated with antigen or antibody.
Reagents required
- Glycine saline buffer: it is a solution used to wash and dilute latex beads and other reagents in order to optimize reaction conditions.
- Test antiserum: The test antiserum comprises antibodies that interact with the specific antigen. It is used to detect the presence of the target antigen in the test sample.
- Blocking buffer: A blocking buffer is a solution that aids in the prevention of non-specific binding during testing. It is used to coat the surfaces of vials or wells in order to reduce background noise.
- Coating antigen: The specific antigen is required for coating the surface of latex beads or other carrier particles. This antigen interacts with the antibodies present in the sample.
Procedure of latex agglutination test
The encapsulation of latex microbeads with pathogen-specific antigens or antibodies is common to both types of latex tests. Agglutination (clumping) is detected when the patient’s cerebrospinal fluid, serum, or urine is combined with coated latex particles in serial dilutions of normal saline.
Latex Coating
- Take 20 µl of latex beads and place them in a 1.5 ml vial. These latex beads serve as carriers for the antibodies.
- Fill the latex beads vial with 40 µl of glycine-saline buffer. The glycine-saline buffer washes and dilutes the latex beads, creating an ideal environment for the coating process.
- Fill the container with rubber beads with 60 µl of the particular antigen. Ensure that the antigen is thoroughly mixed with the latex beads.
- For a period of 2 hours, incubate the vial at 37°C. This incubation allows the antigen to adhere to the surface of the latex beads, resulting in antigen coating.
- After incubation, centrifuge the vial for 10 minutes at 5000 rpm. This process aids in the separation of latex bead-antigen complexes from the supernatant.
- Aspirate and remove the supernatant with caution, being careful not to disrupt the pellet containing the coated latex beads. The pellet comprises latex beads that have been coated with the desired antigen.
- 1 ml of blocking buffer is used to resuspend the pellet. During the succeeding testing procedures, the blocking buffer can be used to inhibit non-specific binding and reduce background noise.
- Centrifuge the vial again for 10 minutes at 5000 rpm. This process aids in the removal of any unbound antigen and improves the coating of the latex beads.
- Repeat the washing process to ensure that any leftover unbound antigen is completely removed.
- Mix in 90 µl of blocking buffer to the pellet. This step ensures that the coated latex beads are suspended in the blocking buffer and ready for the latex agglutination test.
- Incubate the vial at 4°C for an overnight period. This extended incubation period allows for excellent antigen-latex bead binding and guarantees the stability of the coated particles for future testing.
Agglutination Examination
The agglutination test consists of numerous procedures that must be completed in order to observe and understand the results. The agglutination test is performed as follows:
- In a vial, place 200 µl of glycine-saline buffer. This buffer creates an ideal environment for the agglutination reaction to take place.
- To the vial holding the buffer, add 4 µl of the test antisera that has been diluted 50 times. Antibodies specific to the target antigen are present in the test antisera.
- Combine 50 µl of the desired antigen with 50 µl of the diluted test antisera in a separate 1.5 ml vial. To ensure optimal interaction between the antigen and the antibodies, thoroughly mix the contents. Allow this combination to sit at room temperature for 10 minutes to allow the antigen-antibody reaction to take place.
- Pipette 10 µl of pre-coated latex onto a glass slide. As detailed in the previous coating processes, the latex beads need to be coated with the specific antigen of interest.
- 10 µl of the diluted test antiserum (from step 2) should be used to one specific location on the glass slide labelled slide A. This test antiserum comprises antibodies that will bind to the antigen coated on the latex beads.
- Add 10 µl of the antiserum/antigen mixture (from step 3) to another specified spot on the glass slide labelled slide B. This mixture contains both antibodies and antigens, allowing the antigen-antibody reaction to be detected.
- Add 10 µl of glycine-saline buffer to slide C, the third indicated spot on the glass slide. As there is no specific antigen or antibody present in this location, it serves as a negative control.
- Using a toothpick, thoroughly mix the contents of each slide. To eliminate cross-contamination, use a fresh toothpick for each slide.
- Allow the slides to remain undisturbed for 2 minutes after mixing. Observe the reaction on each slide throughout this period.
Result Interpretation
Positive Result
The presence of agglutination, as evidenced by clumps of latex beads, is regarded as a positive result. This shows that the target antigen-antibody response is present. Without the use of a microscope, the agglutination can be seen with the naked eye. The positive result indicates that either the patient’s body created the particular antibody against the pathogen (if the antigen was used by the test) or that the specimen contains the pathogen’s antigen (if the antibody was used by the test). The degree of agglutination can be rated on a scale of 1+ to 4+, with 2+ generally indicating the least amount of visible agglutination in a positive sample.
Negative result
A negative outcome is the absence of agglutination or the creation of clumps. This indicates a lack of the pathogen-specific antigen or antibody being tested for. The lack of apparent agglutination demonstrates that no antigen-antibody response occurred in the tested material.
The interpretation of the data is dependent on the unique antigen-antibody system being studied as well as the specific criteria established by the laboratory or medical expert performing the test. Positive results indicate the presence of the target antibody or antigen in the sample, indicating the presence of an infection or immunological response, whereas negative results indicate the lack of the specific antigen or antibody in the sample.
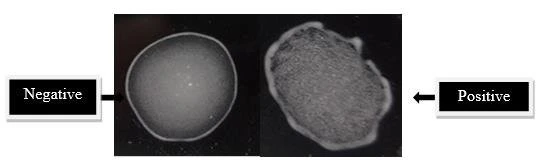
Figure 2: results interpretation of latex agglutination test (Farahmand et al. 2018)
Applications of latex agglutination test
- Latex tests are frequently used to identify Cryptococcus neoformans antigen in cerebrospinal fluid or serum. This aids in the diagnosis of cryptococcal meningitis, a potentially fatal fungal illness.
- To identify specific bacterial infections, the latex agglutination test is used. It may detect bacteria capsular antigens such as Pneumococcus, Haemophilus influenza, and Meningococcus, assisting in the diagnosis and treatment of respiratory and meningococcal illnesses.
- Toxins produced by certain organisms are detected using latex tests, which are constantly being optimized. Latex agglutination tests, for example, can detect Clostridium difficile toxins A and B, which are linked to severe diarrhea and colitis. Furthermore, latex tests are used to detect viral agents such as rotavirus, which is a prevalent cause of gastroenteritis in children.
- C-reactive protein is commonly detected via latex agglutination test. It uses latex particles coated with anti-CRP antibodies. CRP is an inflammatory marker, and high levels suggest that the body has inflammation. This test is useful for assessing and monitoring a variety of inflammatory diseases.
- Antistreptolysin O (ASO) antibodies are detected using the latex agglutination test for antibody detection. ASO antibodies are produced in response to a Streptococcus bacterial infection, specifically group A Streptococcus. This test aids in the identification of post-streptococcal sequelae such rheumatic fever and glomerulonephritis.
- The latex agglutination test can be used to confirm the presence of beta-hemolytic Streptococcus bacteria on culture plates. This aids in the diagnosis and treatment of streptococcal infections, such as strep throat and other similar illnesses.
Advantages of latex agglutination test
- The latex bead size, which is typically 0.8m or greater, enables for easy visualization of the agglutination reaction. The increased particle size enhances the creation of visible clumps, making the test results easier to interpret.
- Because of its simplicity and speed, LAT is one of the most extensively utilized tests. The operation is simple and can be performed in a short period of time. This efficiency is especially useful when speedy results are required for accurate diagnosis and patient management.
- Latex particles used in agglutination tests have been designed to be cross-reactive with other antibodies to the greatest extent possible. This specificity guarantees that test findings are precise and accurate, lowering the possibility of false positives or false negatives. It improves the overall performance and diagnostic utility of the test.
- Latex agglutination assays have shown a great sensitivity to bacterial polysaccharides. The test can identify polysaccharides at extremely low levels, with detection limits as low as 0.1 ng/ml. This sensitivity enables the early detection of infections as well as the accurate monitoring of antigen or antibody levels in a variety of clinical settings.
- Latex agglutination tests are less expensive than other serological testing. They provide a low-cost method for detecting a wide range of analytes, including antigens and antibodies. Because they are more affordable, they are available in a wider range of healthcare settings.
Disadvantages of latex agglutination test
- The circumstances under which latex agglutination processes are performed, such as pH, osmolarity, and ionic concentration, must be precisely controlled. The quantity of binding that occurs between the latex beads and the target antigen or antibody can be affected by changes in these settings. As a result, considerable attention to standardization is required to achieve precise and accurate results.
- Some agglutination procedures demand preparation of specimens before testing. Specimens may, for example, need to be heated to 56°C or treated with ethylene diamine tetra acetic acid (EDTA). These pretreatment processes can take time and complicate the testing process. They may also need additional equipment or reagents, raising the overall cost and effort.
- While latex agglutination tests are often selective for the target antigen or antibody, they can have limits. Cross-reactivity with related antigens or antibodies is possible, resulting in false positive or false negative results. It is critical to evaluate the possibility of cross-reactivity and to use confirmatory testing if needed.
- The visual examination of agglutination patterns is used to interpret latex agglutination test results. This interpretation is subjective and may vary based on the individual performing the expertise and skill of test. Standardized protocols and training are required to reduce inter-observer variability and provide consistent and correct interpretation.
- Certain bodily fluid components, such as rheumatoid factor, can cause false positive results in latex agglutination systems. False positives can disturb the interpretation of test results, perhaps leading to inaccurate diagnoses or needless treatments. It is critical to be aware of potential confounding factors and to consider confirmatory tests where necessary.
Precautions of latex agglutination test
- It is important to thoroughly read and follow the latex test kit instructions. These instructions will cover the specificities of preparing and using the reagents.
- Check that all of the reagents have reached room temperature before beginning the test. This will help to maintain consistency and ensure the optimal performance.
- Make sure the agglutination card or slide is clean and dry before applying the chemicals. Any residue or moisture may affect the results of the tests.
- For precise results, the kit reagents are manufactured at specific concentrations. Avoid diluting any of the reagents because it can compromise the sensitivity and specificity of test.
- The reagents’ characteristics and performance can be altered by freezing them. Keep the kit reagents at the prescribed storage conditions, which are normally within the temperature range specified in the kit instructions.
- Separate mixing sticks should be used for each circle on the agglutination card to avoid cross-contamination. This will help to keep the test’s integrity and prevent false-positive or false-negative results.
- Each reagent in the kit has a distinct function and should not be combined with other reagents. Cross-contamination can occur when reagents are mixed which disturbs the integrity of test.
- Wearing gloves while handling the reagents is recommended to maintain good hygiene and prevent contamination. This reduces the possibility of adding extraneous compounds that could interfere with the test.
Conclusion
The latex agglutination test is a popular and useful method in clinical laboratories for detecting antigens and antibodies. Its benefits include ease of visualization due to the small size of the latex beads, simplicity, quickness, cost-effectiveness, and little cross-reactivity. It has been found to be used in a variety of fields, including the diagnosis of microbial illnesses.
The identification of specific proteins or poisons. It does, however, have significant limitations, such as the demand for thorough standardization, the possibility of false positives induced by specific drugs, and the demand for specimen pretreatment in some circumstances. Despite these limitations, this test remains a significant tool in clinical diagnostics, providing reliable and rapid results for a variety of medical diseases with good implementation and interpretation.
References
- https://vlab.amrita.edu/?sub=3&brch=69&sim=195&cnt=1
- https://miravistalabs.com/medical-fungal-infection-testing/antigen-detection/cryptococcus-latex-agglutination-test/
- https://www.lornelabs.com/news-events/blog/everything-you-should-know-about-latex-agglutination-tests
- https://www.ucsfhealth.org/medical-tests/003334
- https://microbeonline.com/immunology-note/
- Farahmand M, Nahrevanian H, Khalaj V, Mohebali M, Barati M, Naderi S, Zarei Z, Khalili GJIjop. 2018. Assessment of recombinant A2-latex agglutination test (RA2-LAT) and RA2-ELISA for detection of canine visceral leishmaniasis: A comparative field study with direct agglutination test in Northwestern Iran. 13(2): 172.
- Gubala V, Klein R, Templeton DM, Schwenk MJP, Chemistry A. 2014. Immunodiagnostics and immunosensor design (IUPAC Technical Report). 86(10): 1539-1571.