Introduction
Precision is the key to discovering the mysteries of microscopic life forms in microbiology. Serial dilution is a crucial method that enables researchers to produce precise and repeatable results. We shall examine the subtleties of serial dilution and its crucial function in laboratory science in this extensive manual. This article will provide you with the knowledge you need to master the skill of serial dilution, from diluent selection to sophisticated methods and practical applications.
What is Dilution
Dilution is the process of lowering a substance’s concentration by including a diluent or solvent. A series of sequential dilutions are used in serial dilution to produce a range of concentrations for additional analysis.
Diluent Choice
The choice of diluent depends on the nature of the sample and the desired concentration range. A few common diluents are sterile water, saline solution, and growth media made especially for the particular bacterium under investigation.
Dilution Factor Calculation
The ratio of dilution between each step and the desired final concentration is the determining elements in dilution factors. The total dilution obtained during the series can be calculated by multiplying the dilution factors.
Material Required.
Collect the necessary tools, such as
- sterile pipettes
- tubes, or wells
- a micropipette
- a vortex mixer
- a marker for labeling
Procedure
- Based on the initial concentration and the required range of concentrations, choose the dilution ratios. It’s crucial to maintain a balance between getting a good dilution for analysis and making sure the target microorganism can be found.
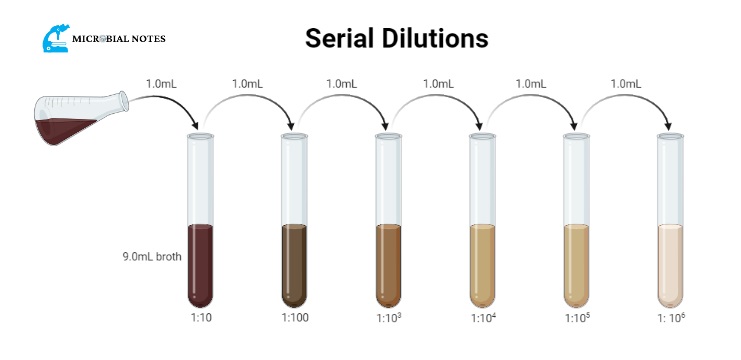
- Take 10 tubes or wells for dilutions add 9ml of diluent in all tubes or 900 µl in all wells
- To prevent confusion during the dilution procedure and subsequent analysis, clearly mark each tube or well. Include important details like the sample’s identification and the dilution factor.
- Transfer 1 ml of the sample into the first tube or 100 µl in well using sterile pipettes. Then, transfer 1 ml of the diluted solution to the following tubes or 100 µl of diluted solution to the following tube wells, making sure that each transfer is properly mixed.
- To achieve homogeneity, the contents must be properly mixed after each transfer. An effective and reliable way to achieve correct mixing is with vortex mixers.
- Always be alert for potential causes of error, such as contamination, improper pipetting, or erroneous mixing, and deal with them immediately.
Calculating Concentrations and Dilution Factors
Dilution Factors Calculation Formula
The most common serial dilutions used are 2 fold dilution and 10 fold dilutions
The dilution factor can be calculated by following the formula
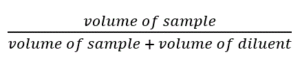
for example as per our procedure we took 1 ml sample and 9ml diluent so
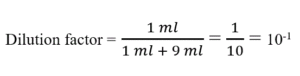
for total dilution factor we need to multiply each dilution factor
suppose
For the first tube, dilution factor = 10-1 (1 ml added to 9 ml)
For the second tube, dilution factor = 10-1 (1ml added to 9 ml)
total dilution factor = previous dilution × dilution of next tube
Total dilution factor = 10-1 x 10-1 =10-2
Calculating Concentration After Dilution
The concentration of the target microbe can be determined when the serial dilution is complete by examining the resulting dilutions, such as by counting colony-forming units (CFUs) or using other analytical techniques.
Types of serial dilutions
Serial Dilution in tubes
The most typical kind of serial dilution, involves a stepwise decrease in concentration to produce a series of dilutions.
Serial Dilution in microplate
High-throughput analysis is made possible by serial dilution carried out on microplates, allowing for the assessment of numerous samples or compounds at once.
Practical Applications
Counting Colony-Forming Units (CFUs)
For the purposes of calculating CFUs in a sample, microbial quantification, and the evaluation of bacterial or fungal growth, serial dilution is crucial.
Maintaining Consistent Cell Cultures
Cell lines are subcultured via serial dilution to keep cell density constant, resulting in ideal growth circumstances and consistent experimental results.
Quality Control Testing
In quality control testing, serial dilution is crucial because it enables the evaluation of the potency, purity, and safety of pharmaceutical goods.
Drug Potency Evaluation
To ensure precise dosing and therapeutic efficacy, drugs potency is assessed by serial dilution.
Monitoring Water Contaminants
Serial dilution makes it possible to identify and measure different contaminants, such as bacteria, viruses, or pollutants, in water samples, providing clean drinking water and environmental protection.
Measuring the Levels of Soil Pollution
In order to measure pollution levels and assess the effects of contaminants on ecosystem integrity and soil health, serial dilution is used in soil testing.
Spectrophotometry
In order to calculate the concentration of chemicals based on their absorbance or optical characteristics, serial dilution is combined with spectrophotometry.
Chromatography
In order to identify and quantify complicated mixtures and separate components, serial dilution is employed in conjunction with chromatography techniques.
PCR, or polymerase chain reaction
To ensure effective amplification and detection of target sequences, serial dilution of DNA or RNA templates is a crucial stage in the PCR process.
Limitations of Serial Dillutions
- A sample propagation error could happen, and the transfer errors result in a transfer that is less precise and accurate. This causes the highest dilution to have the greatest number of errors and the smallest degree of accuracy.
- Because serial dilution is carried out in a stepwise fashion, it requires a longer amount of time, which restricts the method’s effectiveness.
- Serial dilution enables just the reduction of bacteria and cells, not their separation as in other techniques like flow cytometry.
- Aseptic technique specialists and highly trained microbiologists are also needed for this procedure.
Resolving Common Problems
- Preventing Contamination: Apply stringent aseptic methods, such as keeping a clean workspace, utilizing sterile tools, and routinely checking and cleaning work surfaces.
- Variable Dilution Ratios: To preserve consistency in dilution ratios, use accurate pipetting techniques, calibrated pipettes, and accurate volume measurements.
- Excessive or Insufficient Diluting: To prevent excessive dilution that could render the sample undetected or inadequate dilution that fails to attain the target concentration range, be aware of dilution parameters and choose proper dilution ratios.
- Precise Pipetting Methods: Learn the skills necessary to pipette accurately, including accurate pipette calibration, constant hand alignment, and careful control of pipette speed and angle during aliquot transfers.
Future Innovations and Trends
Platforms for serial dilution
The development of tiny serial dilution platforms is enabling precise and high-throughput dilution in small-scale systems. This is made possible by advancements in microfluidics and lab-on-a-chip technologies.
Automation and AI Integration in Dilution Processes
Laboratory operations are being transformed by the integration of robots and artificial intelligence (AI), which provides automated serial dilution systems with improved accuracy, speed, and data processing capabilities.
Conclusion
In conclusion, serial dilution is a fundamental technique in microbiology that enables exact concentration modifications and analysis across a range of fields of study. Understanding serial dilution’s core elements, sophisticated modifications, and practical applications enables professionals and researchers to solve the secrets of germs and chemicals. Accept the flexibility of serial dilution and allow it direct you toward novel insights and developments in scientific investigation and testing.
References
- Tortora, G. J., Funke, B. R., & Case, C. L. (2021). Microbiology: An introduction. Pearson Education Limited.
Willey, J. M., Sandman, K. M., Wood, D. H., & Prescott, L. M. (2019). Prescott’s microbiology (11th ed.). McGraw Hill.
Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA. - https://microbenotes.com/serial-dilution/
- https://en.wikipedia.org/wiki/Serial_dilution