Definition of ELISA
The presence or absence of either specific antibodies or antigens in a sample is measured and detected by the commonly used assay known as ELISA (Enzyme Linked Immunosorbent Assay). This assay is based on the attachment of antibodies or antigens (depending on the target) to a solid surface. Then a detectable signal is generated by adding either enzymes or fluorescent markers. ELISA is commonly used for research purposes, medical diagnostics, and many other fields of science for the quantification and identification of viruses, hormones, proteins, and bacteria.
Must Read: Antigen and Antibody interaction
Principle of ELISA
The principle of ELISA is based on a system of enzymes used for the detection of antigen binding in correspondence to its specific antibody. The attachment of antigen is made on a solid surface like a microtiter plate well. A specific antibody which is labeled with an enzyme is then added to this system. An antigen-antibody complex is formed by the binding of antibodies to the attached antigen.
A chromogenic substrate that is specific to the enzyme is then added after the reaction of antigen and antibody. The addition of substrate results in the change of color, as it is added to act on the enzyme attached to the antigen-antibody complex. In any sample, the amount of antigen is directly proportional to the intensity of the color. The color change can observed visually and then measurement is taken with the help of an ELISA reader or a spectrophotometer.
Enzymes used for ELISA
The requirements of the assay determine which enzyme can be used in ELISA. Enzymes that are commonly used are alkaline phosphatase, peroxidase, and β-galactosidase. Each enzyme is specific to its substrate, and the substrate is also selected based on the enzyme used.
Quantification of antigen or antibody
A standard curve is commonly drawn in ELISA assay for the confirmation of reliability and accuracy. This curve is formed by the measurement of known antigen or antibody concentrations; this can be done by plotting concentrations against the corresponding values of optical density. The quantification of antigen or antibody can be done by the comparison of unknown sample optical density with the standard curve.
Types of ELISA
There are four types of ELISA tests:
- Direct ELISA Test (For Detection of Antigen)
- Indirect ELISA Test (For Detection of Antibody/Antigen)
- Sandwich ELISA (For Detection of Antigen)
- Combined ELISA (For Detection of Antibody)
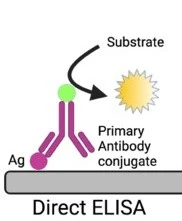
Direct ELISA Test
Direct ELISA is a simple type of ELISA that is based on the direct interaction between the antigen of interest and a single enzyme linked antibody present in the sample.
Procedure of Direct ELISA
The Direct ELISA includes the following steps for the detection and quantification of the antigen in a sample:
- The first step is the attachment of the sample antigen with the microtiter plate wells. The plate has a coating of solid surface which allows the attachment of antigens to the wells.
- With the non-specific interactions, the sample antigen is attached to the wells of the plate. Then, the plate is incubated to ensure efficient antigens binding with the plate surface.
- After incubation, for the removal of unbound and any non-specifically bound antigens, the plate is washed. This washing step is necessary to enhance the specificity of the assay or to minimize any background noise.
- After that, an antigen specific enzyme linked antibody is added in the wells. The antibody is usually conjugated with a suitable enzyme like alkaline phosphatase or peroxidase. The antibody is specific to the antigen and it will bind to it. An antigen-antibody complex will form.
- The plate will be again washed. This step of washing is very important for the removal of antibodies that are excess, not needed and that can cause error in the assay.
- After washing, a chromogenic substrate is added which is specific to the enzyme bound to the antibody. A change in color occurs, as the substrate reacts with the enzyme.
- Finally, color change can be visualized at a specific wavelength. ELISA reader or spectrophotometer can be used to measure the absorbance.
Advantages of Direct ELISA
- This assay takes less time or it is faster if compared to other types of ELISA techniques, as it involves very few steps.
- Less reagents are needed in direct ELISA.
- The chances of errors are less as there is no secondary antibody needed that can result in cross-reaction.
- If quick analysis is needed for the immune response of an antigen, direct ELISA is the best choice.
Disadvantages of Direct ELISA
- The direct ELISA has less specificity as compared to other ELISA techniques and because of the non-specific binding of protein excess background noise can occur.
- For every target antigen or protein, a specific conjugated primary antibody is needed that makes direct ELISA less flexible and may also lessen its versatility.
- Signal amplification is not included in direct ELISA, this can affect the sensitivity of this ELISA method.
Indirect ELISA
This ELISA was developed in 1978 and it is the most commonly used type of Enzyme Linked Immunosorbent Assay (ELISA). Among other types, it is also known as the most popular ELISA technique.
The main goal of indirect ELISA is the detection of antibodies. A primary antibody is involved that can bind to the target antigen specifically. Unlike direct ELISA, this primary antibody is not labeled and does not contain any attached enzyme.
For the detection of primary antibodies, an enzyme-linked secondary antibody is used. The enzyme used can be alkaline phosphatase or peroxidase. This secondary antibody will identify and bind with the primary antibody and form a complex of antibody-antibody.
The procedure of Indirect ELISA
- Known antigens are added to the microtiter plate wells that should be specific to the antibody of interest. Specific antibodies that are present in the sample bind to the known antigens.
- For the removal of any unattached antigens and possible contaminants that can attached to the plate, washing is done. To increase the assay specificity and to minimize the background noise, the step of washing is very important.
- Then test samples (i.e. Serum samples) are added to the wells respectively. If the sample has any specific antibody to the antigens present on the plate, it will attached to it during the period of incubation.
- The plate will be washed again for the removal of unattached antibodies or any contaminant possibly present in the sample.
- An enzyme-linked secondary antibody is then added to the wells that should be specific to the isotype and species of the primary antibody. The function of this secondary antibody is the identification and attachment with the primary antibody (which is already linked to the antigens bound to the plate) and it is conjugated with the enzyme.
- Once again plate will be washed for the removal of non-specific and unbound secondary antibodies.
- After that, a chromogenic substrate antibody is added to the wells that are specific to the enzyme which is linked to the secondary antibody. A change in the color is generated because of the fluorescence signal when the substrate reacts with the enzyme.
- This color change is then observed and quantified either visually or by using an ELISA reader to measure the optical density. In a sample, the amount of specific antibodies is directly proportional to the intensity of the color change or signal generated.
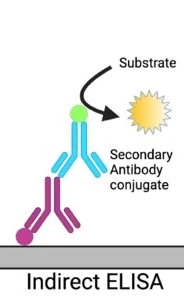
Figure 2: in an indirect ELISA an unconjugated antibody get attached with the target antigen. Then a conjugated antibody bound to it (Salazar et al. 2017)
Advantages of Indirect ELISA
- It is a highly sensitive technique.
- Signal amplification can occur, due to the attachment of multiple labeled secondary antibodies to the primary antibody. This will give a more detectable stronger signal.
- Low levels of antibodies can be detected easily with indirect ELISA because of its enhanced sensitivity due to signal amplification.
- Indirect ELISA is more economical and cost-effective than direct ELISA. Only a few labeled antibodies are required in it. This is because, with multiple primary antibodies, only one labeled secondary antibody can be used.
Disadvantages of Indirect ELISA
- Cross-reactivity is an important drawback of indirect ELISA. This happens because there is a possibility of a reaction between the attached antigen and secondary antibody that can result in background noise. The specification and accuracy of the assay can be affected by this cross-reactivity.
- Indirect ELISA technique is time-consuming as compared to direct ELISA. An additional step of incubation is needed for the binding of the secondary antibody to the primary antibody.
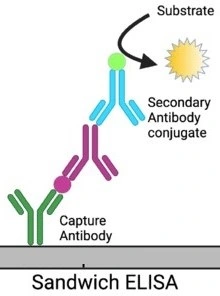
Sandwich ELISA
This assay was developed in 1977 and it is used for antigen detection. Two different types of antibodies are involved in this assay: antibodies attached to the plate surface and enzyme linked secondary antibodies.
In sandwich ELISA, firstly the antigen to be detected get bound to the antibodies that are attached to the surface of the plate, as these antibodies are specific to different antigen epitopes. This will form a structure just like a “sandwich” as the antigen is sandwiched between the surface-attached antibodies and the secondary antibodies.
For the confirmation of accurate results, it is necessary to use matched pairs of antibodies in the sandwich ELISA. To avoid overlapping, antibodies are attached to the surface and detection antibodies must target different epitopes present on the antigen. This specific targeting enables the accuracy in the quantification and detection of the antigen.
Sandwich ELISA is of two types: direct sandwich ELISA and indirect sandwich ELISA. In the direct sandwich ELISA, the enzyme is directly conjugated with the detection antibody. In the indirect sandwich ELISA, the detection antibody used is unlabeled and an enzyme-conjugated secondary detection antibody is added.
Procedure of Sandwich ELISA
- Surface attached antibodies that are specific to the target antigen are added to the microtiter plate wells. These antibodies are fixed to the surface of the microtiter plate and bind to the antigen present in the sample.
- Antibodies that do not bind to antigens are removed by washing the plate carefully. By this other substances present in the sample but that does not bind to the attached antibodies specifically, are also removed. The specificity of the assay was increased by the washing step as well as background noise was reduced.
- The test sample (i.e. serum sample) that contains antigen of interest is then added to the wells. The surface-attached antibodies will bind to the sample antigen and the first layer of the “sandwich” structure will be formed.
- The plate will be washed again just like before for the removal of unbound antigens or some other present in a sample that cannot specifically bind to antibodies.
- Detection antibodies also called enzyme-linked antibodies are then added to the wells. These antibodies bound to the antigen on a different epitope (other than that epitope to which attached antibodies are bound). Detection antibodies can attached to enzymes like alkaline phosphatase, peroxidase, or any other enzyme that can produce a detectable signal.
- The plate will be washed once.
- After washing, a chromogenic substrate linked to the detection antibodies and specific to the enzyme antibodies is added to the wells. The substrate will react with the enzyme and result in the production of the color or any fluorescent signal.
- The color development or fluorescence intensity can be observed visually and can be measured using a spectrophotometer or ELISA reader. In a sample, the amount of antigen present is proportional to the signal intensity.
Advantages of Sandwich ELISA
- This assay is highly sensitive, i.e. it is 2-5 times more sensitive as compared to direct and indirect ELISA.
- This assay is suitable for the low concentration antigens analysis because of the increased sensitivity.
- Two antibodies usage also increases the assay specificity.
- This assay is very flexible and both direct and indirect methods can be used to perform this assay.
- For the examination of complex samples, Sandwich ELISAs are best to use. They don’t need any prior purification and allow direct detection of antigens. This quality makes them useful in many diagnostic and research applications, i.e. cytokine levels measurement in immune response.
Disadvantages of Sandwich ELISA
- For the reduction of cross reactivity between the surface attached antibodies and detection antibodies optimization of antibodies becomes necessary; if standard ELISA kit or antibody pair (already tested) is not available.
- It is a more time consuming procedure as well as it is labor intensive compared to other ELISA formats.
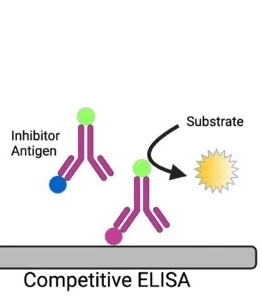
Competition ELISA
Competition ELISA was developed in 1976, it is also known as Inhibition or blocking ELISA and is used commonly for the antibodies detection. The basic principle of this ELISA technique is the competition between enzyme linked secondary antibodies and antibodies of samples (if present) to bind with the antigens.
If the sample has specific antibodies they will bind to the antigens; this will avoid enzyme linked antibodies attachment with the same antigens. Because of this, in the washing step of ELISA enzyme linked antibodies will be washed away. when the substrate is added, there will be no color change, which indicates positive result of test i.e. there is no competition for the specific antibodies. The enzyme linked antibodies may get attached to the antigens, if sample may has not specific antibodies, that will cause change in the color i.e. negative result or the presence of competition.
Concentration of an antigen or antibody can be measured by the competition ELISA in a sample. This is done by the detection of interruption in the output of expected signal. In a sample, the amount of antigen is inversely related to the output signal strength which means if antigen concentration is high then output signal will be weak.
Procedure of Competition ELISA
- In the first step antigen is added to the wells of a microtitre plate. These antigens are attached to the surface of the microtiter plate well.
- When antigens are attached to the plate surface, the plate will be washed for the removal of unattached antigens.
- Then, a serum sample will be added that may have antibodies against antigens attached to the wells.
- The plate will be washed one more time, after the incubation period, for the removal of antibodies that do not bound to the antigens.
- After washing, enzyme linked specific antibodies are poured into the wells. These antibodies may get attached to the antigens that do not bind to the antibodies present in the sample.
- The plate will be washed once again for the removal of unattached enzyme linked antibodies.
- After that, a substrate will be added that will be specific to the enzyme-linked antibodies. The substrate will react to the enzyme and produce a clear detectable signal, commonly change in color.
- The change in color can be seen visually as well as it can be measured by using an ELISA reader or spectrophotometer.
Applications of Competition ELISA
- The competitive ELISA can be used when for the target antigen, only a single antibody is available.
- Unlike the sandwich ELISA, competitive ELISA can be used to detect small antigens that are not able to be attached by two different antibodies.
- There is no need for sample processing for this technique as impure or crude samples can be used directly.
- This assay is more robust or less sensitive to dilutions of sample and matrix effects of the sample as compared to sandwich ELISA.
- Competitive ELISA is more consistent or has less variability and error between duplicate samples.
- This technique shows maximum flexibility as compared to direct, indirect, and sandwich ELISA
Result Interpretations of ELISA tests
- Interpretation of ELISA results is based on both qualitative and quantitative examination of the antigen or antibody that has to be detected.
- Qualitative interpretation has the main focus on the determination of the presence or absence of the antigen. This can be done by observing the change in the color.
- Antigen or antibody concentration can be determined by the quantitative interpretation. The optical density of the microtiter plate wells can be measured by using an ELISA reader or spectrophotometer. The standard curve can be drawn using known antigen or antibody concentrations. Then the unknown sample optical density values are compared to this curve. By the correlation of the optical density and standard sample concentration, the antigen or antibody concentration in the unknown sample can be evaluated.
Application of ELISA test
- ELISA tests have many applications in clinical laboratories as well as in research settings. They are used for the diagnosis of different diseases like HIV, identification of different allergic reactions i.e. food allergies, and detection of autoimmune disorders.
- These tests are also utilized for the development of drug and quality control in the pharmaceutical industry.
- Moreover, there are many specific ELISA kits present for infection detection like tuberculosis (TB), dengue fever, and Hepatitis B.
- Based on ELISA, pregnancy test kits are commonly used for human chorionic gonadotropin hormone detection in blood or urine, which is a reliable and easy method for pregnancy confirmation.
- ELISA helps in the qualitative and quantitative estimation of many hormones and proteins present in the samples.
- This assay plays an important role in the toxins production detection i.e. toxins present in food. So these assays are helpful in testing food safety and toxin screening.
- ELISA is useful in food allergens detection i.e. these assays help in the identification of the specific allergenic present in food products.
Advantages of ELISA
- ELISA protocols are very simple to perform. The assay steps are easy and well established or standardized, which makes them easy to be performed by researchers and laboratory technicians.
- These assays are very specific, which means that they can detect the target analyte accurately from other substances that are present in the sample.
- It helps to analyze multiple samples at a time, which means it is a time-saving technique.
- ELISA does not need any radioactive substances, unlike Radioimmunoassay (RIA), which means this technique is a safer alternative.
Limitations of ELISA
- When we deal with a large quantity of samples ELISA appears to be a labor intensive method. It includes multiple steps, like preparation of sample, coating of plates, period of incubation, plate washing, and analysis of data.
- The enzyme-linked antibodies preparation is a very complicated and time-consuming process. It requires specific conditions as well as optimization. It also includes the conjugation of enzymes with the antibody.
- Antibodies utilized for ELISA techniques are sensitive to conditions of environmental i.e. pH, temperature etc. The antibodies can be degraded with the passage of time which can affect the reliability of the assay.
- Cross reactivity occurs sometimes in this technique, because of this antibodies can bind with substances other than the target. False positive or false negative results can occur because of cross reactivity.
References
- Japp NC, Souchek JJ, Sasson AR, Hollingsworth MA, Batra SK, Junker WMJJoIR. 2021. Tumor biomarker in-solution quantification, standard production, and multiplex detection. 2021.
- Lengfeld J, Zhang H, Stoesz S, Murali R, Pass F, Greene MI, Goel PN, Grover PJBCT, Therapy. 2021. Challenges in detection of serum oncoprotein: relevance to breast cancer diagnostics. 575-593.
- Liu L, Gao Y, Liu J, Li Y, Yin Z, Zhang Y, Pi F, Sun XJBoEC, Toxicology. 2021. Sensitive techniques for POCT sensing on the residues of pesticides and veterinary drugs in food. 107: 206-214.
- Salazar A, Velázquez-Soto H, Ayala-Balboa J, Jiménez-Martínez MC, Salazar A. 2017. Allergen-based diagnostic: Novel and old methodologies with new approaches. In. Allergen. IntechOpen. p.
- https://www.ncbi.nlm.nih.gov/books/NBK555922/