Introduction
Microbiologists get to investigate the fascinating world of microscopic living creatures such as bacteria and viruses. It’s like being an investigator in a microscopic universe where we watch huge battles and unexpected partnerships that keep our bodies healthy. One of the most fascinating stories in the world is about:
How do antigens and antibodies interact?
Consider them to be two distinct molecules that collaborate in an arrangement to defend our bodies. Whether you’re a scientist or just curious, join us on a short trip to see how these tiny heroes collaborate.
Antigen: The Mysterious Invader
Antigens are uninvited guests at a party. They can be bacteria, viruses, or allergies that our immune system must contend with. Consider them as unexpected guests generating a disturbance at the occasion.
Antigen presentation
Assume a big entrance: antigens are presented on the surface of specialized cells. This “presentation” is your body’s way of saying, “We’ve got an intruder, and we need your help!

Antibodies: Our Body’s Secret Weapons
Let’s get to know about antibodies, which are like the stars of our immune system. These Y-shaped proteins are extremely effective at detecting and stopping specific invaders. Consider it a unique key that only fits one single lock, exactly like how each antibody is designed to target a certain opponent
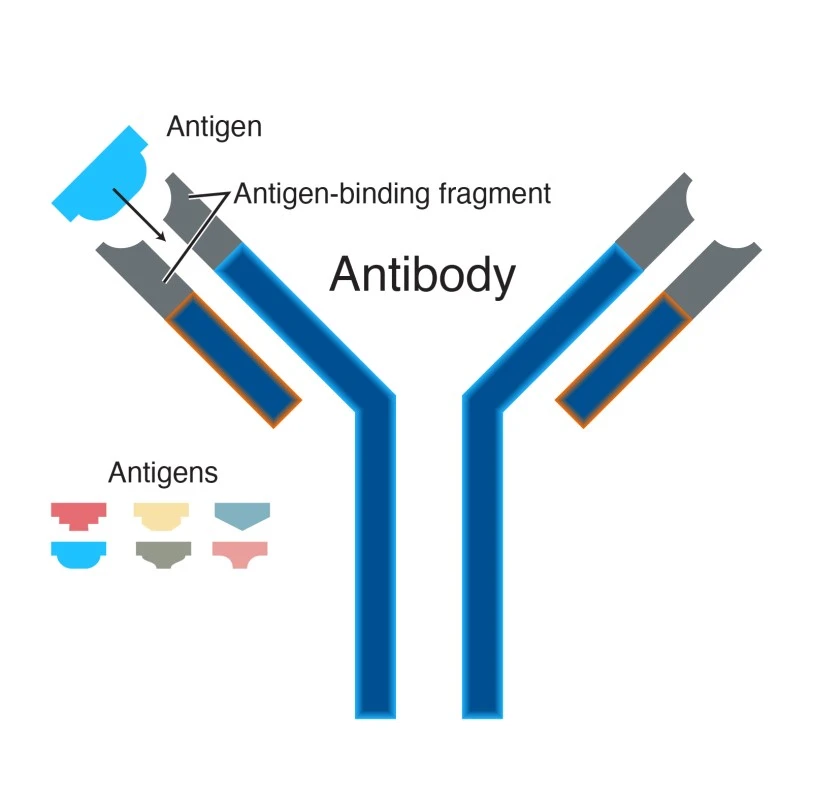
How the lock and key concept relates with antigen antibody interaction ?
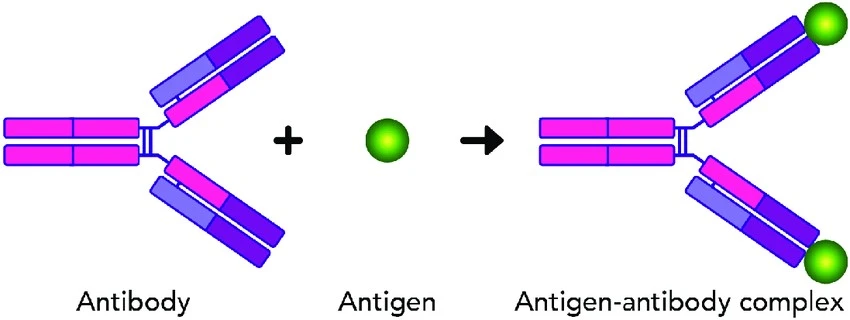
The interaction between antigens and antibodies can be related to a lock and key mechanism. Each antibody has a unique design that fits into the shape of the antigen. When they combine, a signal is delivered to the immune system, indicating that it is time to attack.
Antigen + Antibody ⇄ Ag-Ab complex → Immune Response
The Marvel of Immune Response
Neutralization
Here is the process of how antibodies neutralize the antigen after binding.
1. Antibodies and Antigen
Imagine antibodies as microscopic soldiers in your body. When deadly attackers (such as viruses or bacteria) enter your system, antibodies detect them.
2. Locking on
Antibodies attach to these antigens like invading forces This attachment is quite specific, similar to how a key fits into a lock. Each antibody can only bind to one type of antigen. This is like a security guard catching the bad guy and not letting them escape. Once the antibody attaches to the antigen, it can do a couple of important things:
Blocking harmful properties
The antibody inhibit the antigen from causing harm. It’s like handcuffing the evil guy so they can’t do anything wrong. If the antigen is a virus, for example, the antibody can block it from infecting your body’s cells.
Marking for destruction
The antibody can also attach a tag to the antigen, alerting the immune system’s “police force” to the fact that this is a bad man. This tag assists immune cells in recognizing and attacking the antigen. It’s similar to attaching a “Wanted” poster to the bad man, making it easier for immune cells to locate and remove them.
3. Ultimate Showdown
Consider it like a major showdown in an action movie. The immune system, with its antibodies, is like the story’s hero, battling the villain (the antigen). In this scenario, the immune system wins the battle by stopping the bad person from imposing harm or eliminating them with the support of other immune cells.
Memory formation
But here’s the real magic, the immune system has a photographic memory. When it defeats an invader, it recalls the action, and this memory ensures that if the same antigen ever returns, the immune system will be able to respond effectively and rapidly. This phenomenon is at the heart of vaccinations, our most effective disease-prevention weapon.
The Symphony of Immunity
Fine-Tuning the Response
Interactions between antigens and antibodies are not always straightforward. Depending on the type of threat, the immune system can fine-tune its reaction. This coordination of responses guarantees that each unique attacker is met with a specific security, demonstrating the body’s extraordinary adaptability.
In short, The interaction between antigen and antibody is a stunning example of nature’s defense mechanisms. It is an endless process of exploration and learning. This immune dance gives honor to nature’s beautiful equilibrium, and its research continues to inspire and improve our understanding of health and illness.
Factors affecting antigen and antibody interaction
Antigen-antibody interaction is a fundamental element of the immune system that plays a critical role in immunological responses. Several factors can influence the antigen-antibody interaction, including:
1. Affinity and specificity
The affinity and specificity of the antibody for its antigen are important concerns. Affinity is the strength of binding between an antibody and its antigen, whereas specificity is the antibody’s preference for a certain antigen. A strong and particular interaction results from high affinity and specificity.
2. Epitope and paratope
The epitope is the unique region on the antigen that interacts with the paratope, which is the corresponding region on the antibody. The interaction is greatly influenced by the shape and accessibility of the epitope and paratope.
3. Concentration
The concentration of antigen and antibody in a sample can have an effect on the interaction. Higher antigen or antibody concentrations can enhance the chance of binding.
4. Temperature
The rate and strength of the antigen-antibody interaction can be affected by temperature. Higher temperatures typically result in faster responses, but excessive temperatures can denature proteins and disrupt the interaction.
5. pH
The interaction can be significantly influenced by the pH of the surroundings. The pH ranges of antibodies and antigens are ideal for binding. pH changes can cause changes in the charge and shape of molecules, influencing their interaction.
6. Ionic strength
The interaction can be influenced by the ionic strength of the solution. High salt concentrations can disrupt antigen-antibody electrostatic interactions.
7. Valence
The strength of an interaction can be affected by the number of binding sites on an antibody and the number of epitopes on an antigen. Bivalent antibodies (IgG) contain two binding sites, whereas pentameric antibodies (IgM) have ten, resulting in more powerful interactions.
8. Size and shape
The ability of antigen and antibody molecules to interact is influenced by their size and shape. A strong interaction requires a good match between the paratope and the epitope.
The size and shape of antigen and antibody molecules are important variables in how well they interact. Here’s briefly discuss that
How size and shape affect the antigen-antibody (Ag-Ab) interaction?
Complementary fit
The paratope (the antigen-binding site on the antibody) must have a complementary shape to the epitope (the specific region on the antigen that the antibody recognizes) for a strong interaction to occur. Consider it like a lock and key: a perfect match in shape permits the antibody to bind to the antigen tightly. The interaction may be poor or non-existent if the forms do not match well.
Steric hindrance
The size of the antibody and antigen molecules can cause steric hindrance, which occurs when bulky or obstructive groups on the antigen or antibody prohibit or hinder interaction. A good match between the paratope and the epitope reduces steric hindrance.
9. Post-translational modifications
Glycosylation and phosphorylation can change the shape and charge of antigens and antibodies, affecting their interaction.
10. Cross reactivity
Cross-reactivity occurs when an antibody recognizes and binds to antigens that share structural similarities with the target antigen. This can result in unwanted and generally non-specific binding, which can have a number of effects:
Non specific binding
Cross-reactivity can cause antibodies to attach to non-target antigens, resulting in false-positive immunoassay or experiment findings. This could compromise the assay’s specificity.
Reduced sensitivity
Cross-reactivity may result in competition for binding sites between the target antigen and structurally related molecules in some situations. This can limit the assay’s sensitivity, making it less capable of detecting lower amounts of the target antigen.
False interpretation
Researchers must exercise precaution when interpreting results, particularly when cross-reactivity is known to occur. Incorrect conclusions can result from failing to account for cross-reactivity.
Diagnostic challenges
Cross-reactivity can compromise test accuracy in clinical diagnosis. A diagnostic test for a specific pathogen, for example, may cross-react with other, non-target infections, potentially leading to a misdiagnosis.
11. Solubility and aggregation
Antigen and antibody solubility can influence their interaction. Protein aggregates that are insoluble may not interact effectively.
12. Time
The time of antigen and antibody exposure can influence the interaction. Longer incubation durations can result in more powerful binding.
Understanding these characteristics is critical in a variety of applications, such as immunology, diagnostics, and research, because they can affect the sensitivity, specificity, and overall success of antigen-antibody interactions in assays and research.
Chemical bonds responsible for Antigen-Antibody interaction
The relationship between the Ab-binding site and the epitope is entirely based on non-covalent bonding, similar to how proteins attach to their cellular receptors or enzymes bind to their substrates. The binding is reversible, and strong ionic strength or severe pH can prevent or dissociate it. Ag-Ab binding involves the following intermolecular forces:
a) Hydrogen bonding
Hydrogen bonds form between the amino acid particles of the antibody’s variable region (complementarity-determining regions, CDRs) and the antigenic determinant’s epitope. These bonds are rather weak, yet they add to the uniqueness and affinity of the contact.
b) Electrostatics interaction
Charged amino acid residues on the antibody and antigen interact electrostatically. Positively charged amino acids, for example, may interact with negatively charged amino acids on the antigen.
c) Van der wall forces
Van der Waals forces are attractive forces caused by transitory changes in the electron distribution. They can form between nonpolar regions of the antibody and antigen and contribute to the complex’s stability.
d) Hydrophobic interaction
When nonpolar portions of the antibody and antigen remain separate from the surrounding aqueous environment, hydrophobic interactions can occur. This can help to keep the binding in place.
These non-covalent connections determine the specificity and affinity of the antigen-antibody interaction, enabling antibodies to recognize and attach to their target antigens with high accuracy and selectivity.
Types of antigen and antibody interaction
Antigen antibody interactions are basically of 2 types.
In vivo ( Ag-Ab interaction occurs naturally):
- Agglutination
- Opsonization
- Neutralization
- Complement activation
- Allergic reactions
- Immobilization
- Cross-linking
- Immune complex formation
In vitro (Ag-Ab interaction occurs in artificial or lab environment):
- Immunoprecipitation
- ELISA (Enzyme-Linked Immunosorbent Assay)
- Western blotting
- Immunofluorescence
- Immunohistochemistry
- Flow cytometry
- Radioimmunoassay
- Immunodiffusion
These interactions are examined by researchers in both laboratory settings (in vitro) and within living creatures (in vivo) to better understand immune responses, diagnose diseases, and undertake microbiology and immunology research.
1. Agglutination
Read in detail : Agglutination Tests – Its types, Applications with examples
Agglutination is a type of antigen-antibody interaction in which antibodies bind to numerous antigens on the surface of microorganisms like bacteria or viruses. This binding causes apparent clumps or aggregates of these particles to form. Agglutination is a critical mechanism in the immune response that can occur both in vivo (inside the body) and in vitro (in the laboratory).
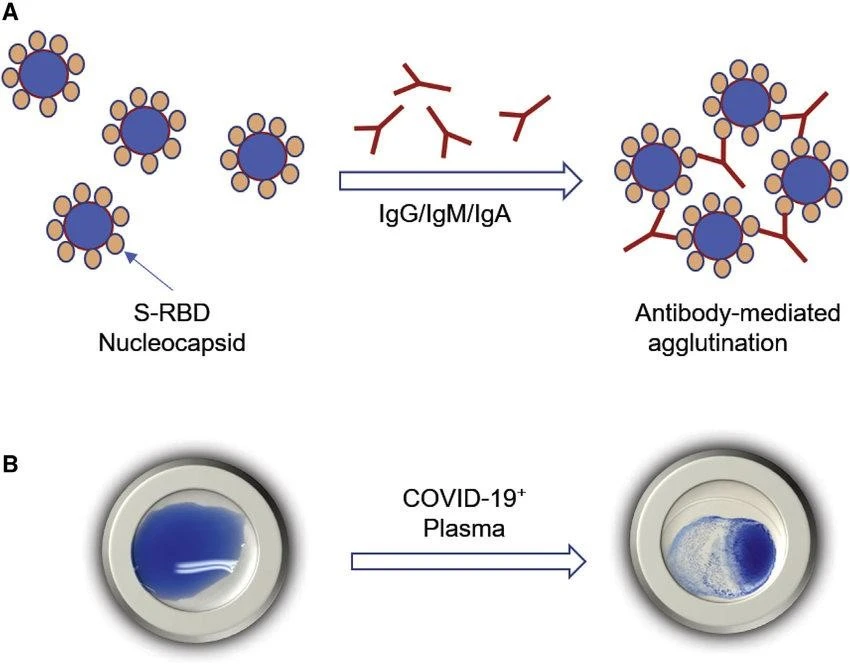
2. Opsonization:
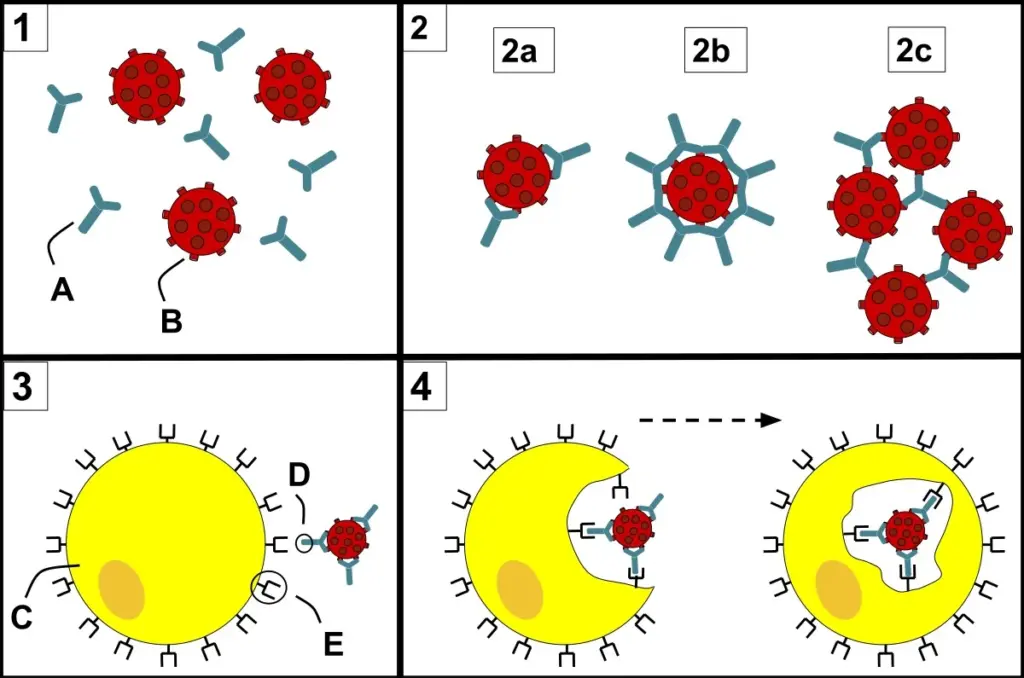
Opsonization is a form of antigen-antibody interaction that is essential in the immune system’s defense against infections, specifically bacteria. In opsonization, antibodies bind to the surface of pathogens such as bacteria, enhancing immune cell phagocytosis. This procedure dramatically improves the efficiency with which infections are removed from the body. Here’s a brief explanation:
Antibody binding
Opsonization starts with antibodies (immunoglobulins or Ig) attaching to specific antigens on pathogen surfaces. Each antibody molecule contains two Fab (antigen-binding) regions capable of interacting with antigens. Because antibodies recognize and bind to specific epitopes on the pathogen’s surface, this contact is very specific.
Enhancement of phagocytosis
Once antibodies bind to a pathogen, they effectively “tag” it for elimination by immune cells, specifically phagocytes such as macrophages and neutrophils. Phagocytosis is the process by which an immune cell engulfs and digests a pathogen.
Recognization by phagocytosis
Phagocytes have receptors for antibodies’ constant (Fc) region. These receptors recognize and bind to the Fc sections of antibodies when they are linked to a pathogen. This contact sets off a series of signals within the phagocyte, activating it and boosting phagocytosis.
Uptake and destruction
The phagocyte engulfs and encases the opsonized pathogen in a vesicle known as a phagosome. The phagosome subsequently combines with lysosomes to form a phagolysosome, in which the pathogen is exposed to damaging enzymes, antibiotic compounds, and oxidative processes, which eventually lead to its disintegration and elimination.
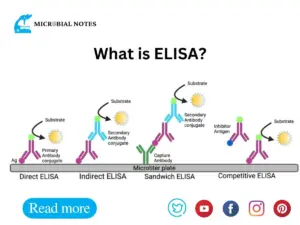
3. ELISA
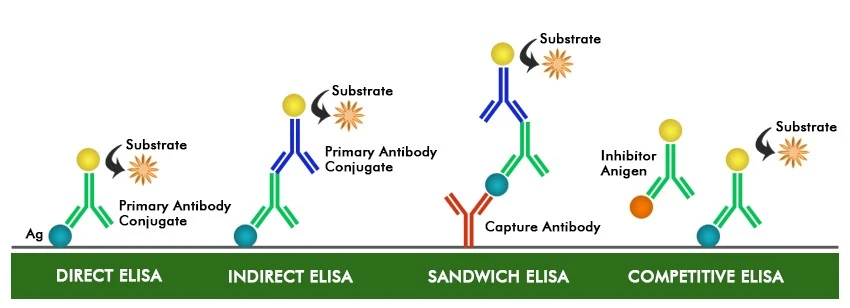
The Enzyme-Linked Immunosorbent Assay (ELISA) is a laboratory technique for detecting and quantifying specific antigens (Ag) or antibodies (Ab) in a sample. The ELISA assay is a useful and sensitive assay that is widely used in clinical diagnostics, research, and other sectors. It is based on antibody-antigen interaction and can be used to identify the presence of certain antibodies or antigens in a sample.
ELISA can be adapted for various purposes :
Direct Elisa
A labeled antibody is used to detect the presence of an antigen.
Indirect Elisa
Detects the presence of an antibody using a secondary antibody that has been labeled.
Sandwich ELISA
Two antibodies are used to sandwich the antigen of interest for detection.
Competitive ELISA
The competition for binding to a restricted number of antibody binding sites between the sample’s antigen and a labeled antigen.
4. Neutralization
Read in Detail: Neutralization Test | Its Principle types and uses
In the antigen-antibody interaction, neutralization occurs when antibodies bind to antigens and prevent them from causing harm. This binding can prevent pathogens like viruses or poisons from acting, neutralizing their effects and assisting in immune defense.
5. Complement Fixation
Complement fixation is the process by which antibodies activate the complement system, a collection of proteins in the blood. When antibodies connect to antigens, they can activate the complement cascade, causing numerous complement proteins to be activated.
This activation can lead to the synthesis of a membrane attack complex, which may enter pathogen cell membranes and destroy them. Complement fixation boosts the immune response by increasing inflammation and enabling the clearance of antibody-bound pathogens.
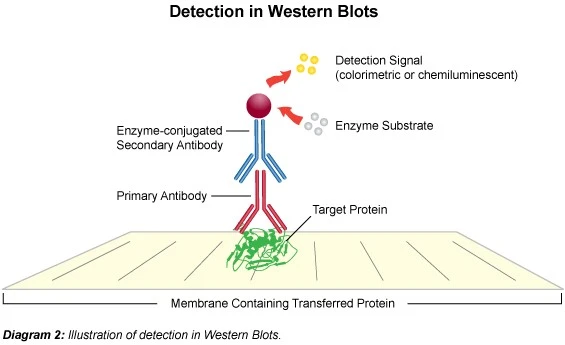
6. Western blotting technique
Western blotting involves isolating proteins, transferring them to a membrane, and employing antibodies to detect a specific protein. Following protein separation, the membrane is incubated with a primary antibody directed at the protein of interest, followed by a secondary antibody coupled to an enzyme or fluorescent. This permits the target protein to be visualized and quantified on the membrane.
Applications of Antigen-Antibody interaction
- The most prevalent application is the determination of blood groups, often known as blood typing.
- This idea supports rapid diagnosis test kits used for pregnancy testing as well as the detection of several diseases such as malaria and dengue. They only need a few minutes to complete the tests.
- Serological detection antigen quantification, etc.
- Detection of protein presence or absence in serum.
- To investigate the characteristics of various immunodeficiency disorders.
- Confirmatory tests for infections, such as Western Blotting for HIV infection, are performed.
Limitations of Antigen Antibody interaction
- Higher skills and equipment are required, which are not often available in some poor or undeveloped countries. As a result, these procedures are rarely applied in such areas.
- Low-density parasitemia (200 parasites/L) is not reliably detected by RDTs.
- In different cases of antibody detection, it is difficult to distinguish between early and late infections since antibodies stay in our blood for a long time after the infection has been cured.
References and resources
- Tortora, G. J., Funke, B. R., & Case, C. L. (2021). Microbiology: An introduction. Pearson Education Limited.
- Atlas, R. M. (2010). Handbook of Microbiological Media. CRC Press.
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA.
- Parija S.C., (2009), Textbook of Microbiology and Immunology, 2nd edition, Elsevier, a division of Reed Elsevier India Private Limited, pg. 94-115
- https://en.wikipedia.org/wiki/Antigen-antibody_interaction