Definition
The Widal test is a serological agglutination test utilized to diagnose Salmonella typhi and Salmonella paratyphi enteric fever. It detects specific antibodies produced in the patient’s serum in response to these bacteria.
Introduction to Widal test
Georges Fernand Isidor Widal, a French physician and bacteriologist, developed the Widal test in 1896. Originally used to diagnose Salmonella paratyphi B infection, the test was later refined and used to diagnose various forms of enteric fevers.
The Widal test works on the basis of the antigen-antibody agglutination reaction. The test uses purified antigens (that are commercially available) of Salmonella strains that cause enteric fever. The serum of the patient is analyzed against these antigens, and the test is considered positive if visible agglutination occurs.
The cell wall of Salmonella typhi and flagella include O and H antigens, respectively. The flagella of Salmonella paratyphi A and paratyphi B contain AH and BH antigens, respectively. The Salmonella paratyphi O antigen is not utilized in the test because it interacts with the Salmonella typhi O antigens.
When antigens enter the immune system of the host, they stimulate the development of specific antibodies. After 6-8 days of infection, these antibodies are detectable in the serum. Through an agglutination reaction, the Widal test recognizes these specific antibodies.
The Widal test is an agglutination test that detects antibodies that are formed in reaction to the causative agents of typhoid and paratyphoid infections, Salmonella typhi and Salmonella paratyphi A, B, and C. It is commonly used as an important technique for determining the underlying cause of many febrile disorders, particularly in endemic areas.
Objectives of Widal test
- The Widal test is designed to detect the presence of particular serum antibodies against Salmonella typhi and paratyphi. The test utilizes to diagnose enteric fever caused by these microorganisms by assessing agglutinating serum antibodies.
- The presence of antibodies shows that the patient has been infected with or has been exposed to Salmonella typhi or paratyphi. The test aids in the diagnosis of enteric fever and distinguishes it from other febrile disorders.
- The Widal test is very beneficial in locations where enteric fever is common, and it is an important diagnostic technique for determining the cause of the illness. The test results help healthcare practitioners make accurate diagnoses, initiate appropriate treatment, and use preventive measures in place.
Principle of Widal test
The Widal test works on the concept of antigen antibody agglutination or interaction response. The serum of the patient is combined with the killed bacteria suspension, particularly Salmonella which has O, H, AH, and BH antigens. An obvious clumping reaction (agglutination) occurs if the patient possesses homologous antibodies against these antigens.
Antibodies against Salmonella typhi and paratyphi O and H antigens are abundant in the serum of patients infected with these types of bacteria during enteric fever. Antibody levels peak 6-8 days after infection. The serum of the patient is taken and combined with Salmonella typhi antigens O and H, as well as Salmonella paratyphi antigens AH and BH. Antibodies in serum will agglutinate to the corresponding antigens, resulting in clear clumping.
When someone consumes Salmonella-contaminated food, the antigens of bacteria enter their body. Antibodies against these antigens are then produced by the immunological system. The presence of antibodies produced by the body in response to a specific pathogen or antigen is detected by this agglutination test. The patient’s serum will include antibodies that react with and agglutinate to the Salmonella antigens in the Widal test in the case of typhoid fever.
Requirements for Widal test
- Blood sample or serum: A fresh serum sample or blood sample from the suspected patient is needed for testing. It is important to note that the serum should be fresh and stored at a temperature between 2°C and 8°C. Heating or inactivation of the serum should be avoided to maintain the integrity of the antibodies.
- Widal test kit: The kit should contain vials with stained Salmonella antigens. Specifically, it should include the following antigens:
- O antigen of Salmonella typhi
- H antigen of Salmonella typhi
- AH antigen of Salmonella paratyphi A
- BH antigen of Salmonella paratyphi B
- Positive control: A positive control sample is included in the kit to validate the test and ensure accurate results.
- Widal test card or slide: A Widal test card, which is specifically designed for the test, is commonly used. However, a glass slide can also be used as a substitute.
- Applicator stick: An applicator stick is required for mixing and transferring the serum and antigens during the test.
Procedure of Widal test
Widal testing is done in two ways: by quick slide agglutination and by tube agglutination. Agglutination on slides can be used to perform both qualitative and quantitative tests. Although the tube approach is more sensitive and accurate than the slide method, the slide method is more widely used.
Antigen preparation for Widal test
- A 24-hour broth culture or agar suspension in saline is made. 0.1% formalin is poured into this culture. Formalin works as a fixative, retaining the bacteria’s antigenic characteristics. The culture treated with formalin is incubated for a set specified time.
- The bacteria are grown on phenol agar at a 1:800 dilution. The phenol in the agar restricts the growth of flagella, which is responsible for the O antigens. This step guarantees that there are no O antigens in the final suspension.
- Widal antigens are made from standard uniform strains of the target organism. The strain mostly used in Widal testing is S. Typhi 901. These strains were chosen because of their well-documented antigenic characteristics.
- The selected strain growth is emulsified in a small volume of saline. After that, the bacterial solution is combined with 20 times its volume of alcohol, usually ethanol.
- The prepared mixture is then heated for 30 minutes at a temperature ranging from 40°C to 50°C. The heat treatment destroys the bacteria while leaving the antigens intact. The mixture is centrifuged after heating in order to separate the bacterial particles along with other impurities from the antigen solution.
- A preservative, such as chloroform, is added to ensure the stability of antigen and lifespan. Chloroform aids in the prevention of microbial development and the preservation of the antigenic characteristics of the solution. Finally, dyes are added to the antigen suspension to facilitate identification during diagnostic procedures. These dyes aid in the differentiation of various antigens and improve visibility.
- This test utilizes the Widal test card. This is the most frequently used test, and it is available in two different forms:
Qualitative Slides Test
The Qualitative Slide Test is a method for determining the presence or absence of Salmonella antigens linked to enteric fever. This test is easy and quick, however, it does not allow for the determination of antigen levels. The following steps are taken to carry out the test:
- The reaction circles on the Widal test card are labeled O, H, AH, BH, PC (positive control), and NC (negative control).
- Each reaction circle labeled O, H, AH, and BH receives a drop of the serum sample. These reaction circles represent Salmonella antigens.
- A drop of the positive control reagent is placed in the reaction circle labeled PC, and a drop of the negative control reagent is placed in the reaction circle labeled NC. These controls serve as points of reference for understanding the test results.
- A drop of antigen solution H from the Widal test kit is poured into the positive and negative control reaction circles.
- In each reaction circle, a drop of antigen solution O, H, AH, and BH is poured into the sample serum. Salmonella antigens are present in these antigen solutions.
- The serum and solutions of antigen in each reaction circle are uniformly mixed with an applicator stick. This ensures that the antigens make proper contact with the serum.
- To allow the reaction mixture to circulate within the reaction circles, the slide is gently moved or spun in a circular motion. This motion helps identify any macroscopic agglutination, which suggests the presence of antibodies against Salmonella antigens.
Interpretation of result
Positive Test: If agglutination, which emerges as clumping or aggregation of particles, appears within a minute of combining the antigen solution and the patient’s serum in the reaction circles, the test is considered positive. This reveals the presence of Salmonella antigens in the patient’s serum, indicating a probable Salmonella infection.
Negative Test: If no agglutination appears within a minute, the test is termed negative. This means that there are no detectable Salmonella antigens in the serum of the patient, indicating the lack of a current Salmonella infection.
Quantitative Slide Test
The Quantitative or Semi-quantitative Slide Test is a diagnostic procedure for determining the concentration of antigens in a serum sample. When the qualitative test, which identifies the presence or absence of antigens, provides a positive result, this test is done. The following are the steps involved in carrying out the test:
- On the slide, a drop of normal saline is placed in the first reaction circle. This acts as a control and helps to provide a baseline for comparison.
- The remaining reaction circles on the slide are filled with 5, 10, 20, 40, and 80 µl of the serum sample (test sample), each in its own reaction circle. The varying serum sample quantities allow for a semi-quantitative measurement of antigen levels.
- In each of the reaction circles containing the test samples, a drop of the antigen suspension that gave a positive result (agglutination) in the qualitative test is added. The antigens of interest are present in this positive antigen suspension.
- Using an applicator stick, mix the components of each reaction are mixed thoroughly. This ensures that the antigen suspension and test samples make good contact.
- The reaction mixture is allowed to circulate within the reaction rings by gently shaking or rotating the slide in a circular manner. This movement makes it easier to detect any macroscopic agglutination, which indicates the presence of antigen-antibody interactions.
Interpretation of results
Within a minute, the test results are visible. The occurrence of macroscopic agglutination, apparent as particle clumping or aggregation, implies a positive outcome. The extent of agglutination is then visibly compared across reaction circles holding varying amounts of test samples. This gives a semi-quantitative evaluation of the antigen levels in the serum sample.
The antibody titer is calculated by selecting the maximum serum dilution that causes a positive reaction, as shown by visible agglutination. The test findings are interpreted depending on the dilution and antibody titer.
For example, if agglutination is found in the fourth reaction circle with 20 µl of serum sample, the antibody titer is recorded as 1:80. This means that a serum sample could be diluted up to 1:80 and still exhibit noticeable agglutination.
A significant antibody titer is one that is greater than 1:80. This indicates that agglutination requires a dilution of 1:80 or higher. Because antibody titers differ based on the disease or condition being examined, the significance of the titer may differ in different clinical circumstances.
The Quantitative (Semi-quantitative) Slide Test provides an approximate estimate of antigen levels in a serum sample. It should be noted, however, this method provides a relative measurement and may not produce precise quantitative data. Alternative approaches, such as enzyme-linked immunosorbent assays (ELISA) or other specialized laboratory procedures, may be required for more accurate and exact assessment of antigen levels.
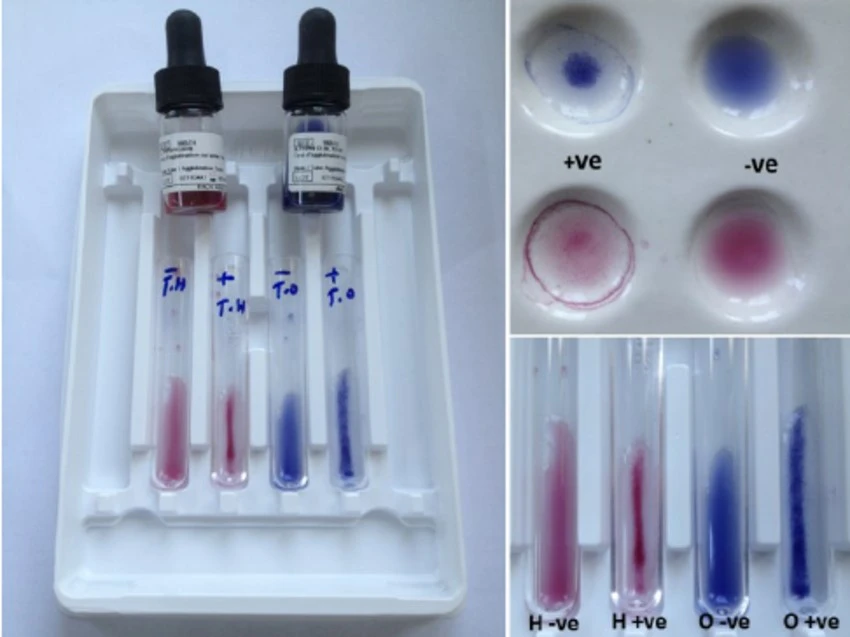
The Quantitative Tube Agglutination Test,
This test is commonly known as the Quantitative Tube Test, is a serological test used for detecting antibodies and measuring their titer in a serum sample. Although many types of tubes were originally used for this test, modern approaches frequently use Khan tubes or simple test tubes. The test is carried out as follows:
- Put four sets of dry and clean Khan Tubes, each set having eight tubes. Label the tubes in each set with the numbers 1 to 8.
- 1.9 mL of 0.85% isotonic sterile saline solution is added to tube 1 of each set. Add 1.0 ml of isotonic sterile saline solution to the remaining tubes (2–8) in each pair.
- Fill tube 1 of each set with 0.1 mL of serum sample and thoroughly mix it with saline solution.
- Transfer 1 ml of diluted serum from tube 1 of each set to tube 2 of the same set. Repeat this repeated dilution process until you reach tube 7. Dispose of 1 ml of the dilution in tube 7 of each batch (i.e. do not transfer to tube 8).
- Add 1 drop of Widal antigen-O to each tube in set 1, 1 drop of Widal antigen-H to each tube in set 2, 1 drop of Widal antigen-AH to each tube in set 3, and 1 drop of Widal antigen-BH to each tube in set 4. These antigens are corresponding to particular Salmonella antigens.
- In each tube, thoroughly mix the materials, cap the tubes, and incubate these at 37°C for about 18 hours (or overnight). This gives time for antigen-antibody interaction.
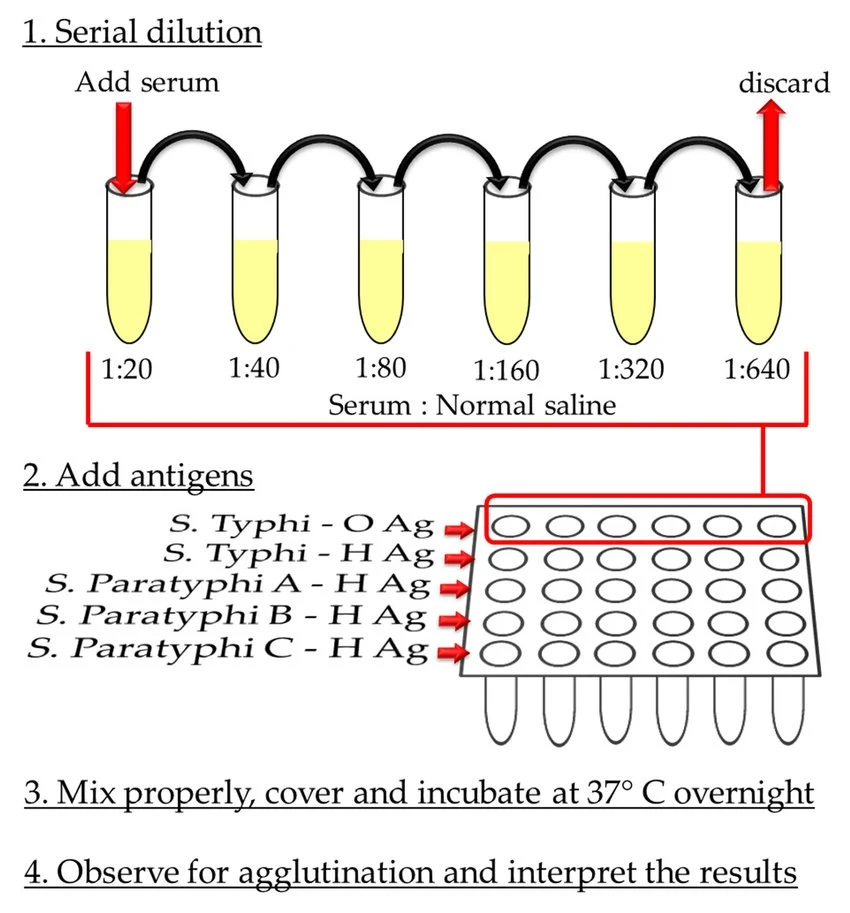
Interpretation of results
Examine tubes for agglutination after the incubation time. Agglutination presents itself as the clumping of particles at the bottom of the tubes. For example, if agglutination is observed up to the fifth tube, which has a dilution of 1:320, the antibody titer is reported as 1:320. To see agglutination, a dilution of 1:320 or greater is required.
A significant antibody titer is typically larger than 1:80. To see agglutination, a dilution of 1:80 or higher is required. Because antibody titers differ based on the individual disease or condition being examined, the significance of the titer may differ in different clinical circumstances.
It is worth noting that the eighth tube acts as a negative control. It contains no serum and is used as a control to ensure that no agglutination occurs in the absence of antibodies.
The Tube Agglutination Test can offer information on the presence of antibodies and their titer in a serum sample by evaluating the presence or absence of agglutination in the tubes. The higher the dilution at which agglutination is carried out, the greater the antibody titer against the specific Salmonella antigens under consideration. This test is useful for detecting specific diseases, such as enteric fevers caused by Salmonella strains, as well as monitoring immunological responses of an individual to these infections.
Applications of Widal test
- This test is generally used as a quick diagnostic test for enteric fever, including typhoid fever and paratyphoid fever. The test confirms the existence of Salmonella infection and helps in the diagnosis of enteric fever by finding particular antibodies in the serum of the patient.
- The Widal test is a quick screening test for detecting instances of typhoid fever in regions where it is endemic and where enteric fever is common. It enables rapid identification of individuals who may require additional diagnostic assessment or therapy, assisting in illness management early on.
- It is effective against both Salmonella typhi and Salmonella paratyphi, which are the major causes of enteric fever. It aids in the detection of antibodies that are unique to these bacteria, which aids in the diagnosis and distinction of typhoid and paratyphoid fever.
- Widal test is also used to identify the serovar or specific strain of Salmonella that is the main cause of enteric fever. It can distinguish between Salmonella typhi, Paratyphi A, and Paratyphi B serovars. This knowledge is essential for proper infection treatment and management.
- It is regarded as a reliable option for detecting enteric fever in instances where bacterial culture facilities are not readily available or acquiring a bacterial culture is difficult. It gives important diagnostic information without requiring specialized laboratory equipment or methodologies.
Advantages of the Widal test
- This test is reasonably inexpensive when compared to more modern laboratory techniques. It requires less equipment and money, making it suitable for use in healthcare facilities with low resources.
- The Widal test is a quick way to detect enteric fever, especially in areas where the disease is common. This enables faster diagnosis and therapy beginning, resulting in better patient care.
- In many areas where enteric fever is common, this test is widely available and consistently utilized. It is an established serology test that has been in use for many years, and its accessibility adds to its utility in clinical practice.
Disadvantages of the Widal test
- Vaccinated or already infected persons may generate false-positive results, resulting in incorrect test interpretation.
- Cross-reactivity with antibodies developed against different Salmonella species could result in false-positive results.
- Baseline titers in the local healthy population should be considered when interpreting Widal test findings. However, baseline antibody levels might vary over time and in different regions, making stable cutoff levels for proper interpretation difficult to establish.
- When compared to other rapid diagnostic procedures, quantitative tube tests for antibody titers require more time for incubation and observation.
- Antibody responses can be disrupted by factors such as acute hypoproteinaemia, early antibiotic treatment, or typhoid relapse, resulting in false-negative results.
- Variance in the results and reading of Widal tests between laboratories may affect the accuracy of the test and consistency.
- Antibiotic treatment may suppress antibody responses, resulting in false-negative results. Individuals with immunological diseases may also have low levels of antibody production, which contributes to false-negative results.
- Agglutinins, the antibodies discovered by the Widal test, usually are apparent in the serum 6-8 days post infection and can last up to 4 weeks. As a result, the test has limited sensitivity and effectiveness whether administered before or after the first week of infection.
- The clinical importance of agglutinin titers varies by geographical area and individual, making it difficult to develop generally appropriate cutoff limits for interpretation.
- Widal test cannot distinguish between a current infection and a past infection or typhoid immunization. This can make assessing the real disease status challenging.
- This test can take a long time to determine antibody titers. In certain circumstances, it may already be too late to begin an antibiotic regimen by the time the diagnosis is established.
- It is a preliminary test that requires further confirmation in order to determine an enteric fever diagnosis. The Widal test alone may result in a misdiagnosis.
- Cross-reactivity with the antibodies such as typhus, malarial parasites, and non-enteric Salmonella infections, is possible. This cross-reactivity has the potential to produce false-positive outcomes.
Conclusion
In summary, the Widal test is a commonly used agglutination test for identifying Salmonella Typhi and Salmonella Paratyphi A, B, and C enteric fever. It uses commercially available antigens to identify specific antibodies generated in response to these microbes. The results are interpreted on the basis of agglutination, which indicates the presence of Salmonella in the serum sample.
References
- Alhaj-Qasem DM, Al-Hatamleh MA, Irekeola AA, Khalid MF, Mohamud R, Ismail A, Mustafa FHJD. 2020. Laboratory diagnosis of paratyphoid fever: Opportunity of surface plasmon resonance. 10(7): 438.
- Zorgani A, Ziglam HJTJoIiDC. 2014. Typhoid fever: misuse of Widal test in Libya. 8(06): 680-687.
- https://www.testing.com/tests/widal-test/
- https://www.portea.com/labs/diagnostic-tests/widal-test-typhoid-test-typhidot-124/
- http://www.ncbi.nlm.nih.gov/pubmed/7342378
- https://cvi.asm.org/content/19/11/1833