What is Plate count method?
For obligate and anaerobic bacteria, the Plate count method is the most frequently utilized technique. This method uses repeated dilution and colony forming unit (CFU) counts to isolate microbial colonies. With this method, the liquid sample is added to the petri dish before the agar medium solidifies (this method is called the pour plate method). colonies form inside and on top of the surface, After the medium solidification. The confluent colonies, on the other hand, are the ones that are growing inside the medium.
What is the objective of the Plate count Technique?
- To separate the microorganisms that are in suspension.
- Colony forming units (CFU) per milliliter can be used to estimate how much viable microbial population is present.
- From a mixed population, to separate the purest cultures of microorganisms
- to separate bacteria from several microorganisms to study the traits of colonies.
Principle
The Plate count method is based on the idea that each viable bacterium will proliferate and form a distinct colony when an agar media containing microorganisms is incubated. This procedure involves properly mixing a particular volume, often 1 mL, of the serially diluted liquid sample with around 15 mL of molten agar medium at a temperature of 40–45°C (less than 50°C) in a Petri plate.
Allow the medium to solidify, then it is normally incubated for 24 to 48 hours at 37°C. After incubation, the sample’s viable microorganisms will form separate colonies both inside and on the surface of the medium. The visible colonies can be counted, and the following formula can be used to determine CFU/mL.
| CFU/mL=Total number of colonies obtained x dilution factor/volume of specimen used. |
What are the requirements for the Plate count Method?
- Sterilization equipment: Autoclave or sterilizer to sterilize all the equipment, materials, and media before use.
- Dilution blanks: Glass test tubes or sterile pipette tips for preparing serial dilutions of the sample.
- Plate count agar: Petri dishes containing agar medium for culturing microorganisms. The type of agar used will depend on the microorganisms being tested.
- Inoculating loops: Metal loops are used to transfer the diluted sample onto the agar plates.
- Colony counter: A device used to count the colonies that form on the agar plates.
- Incubator: A device used to provide optimal conditions for the growth of microorganisms, including temperature and humidity control.
- Pipettes: Single-channel or multi-channel pipettes for dispensing precise volumes of liquids during the dilution process.
- pH meter: A device used to measure the pH of the media to ensure that it is within the optimal range for microbial growth.
- Sterile water: Water that has been sterilized and used for diluting the sample and preparing the media.
- Sterile media: Liquid or solid media used for culturing microorganisms. The type of media used will depend on the microorganisms being tested.
What is the procedure of the Plate count Method?
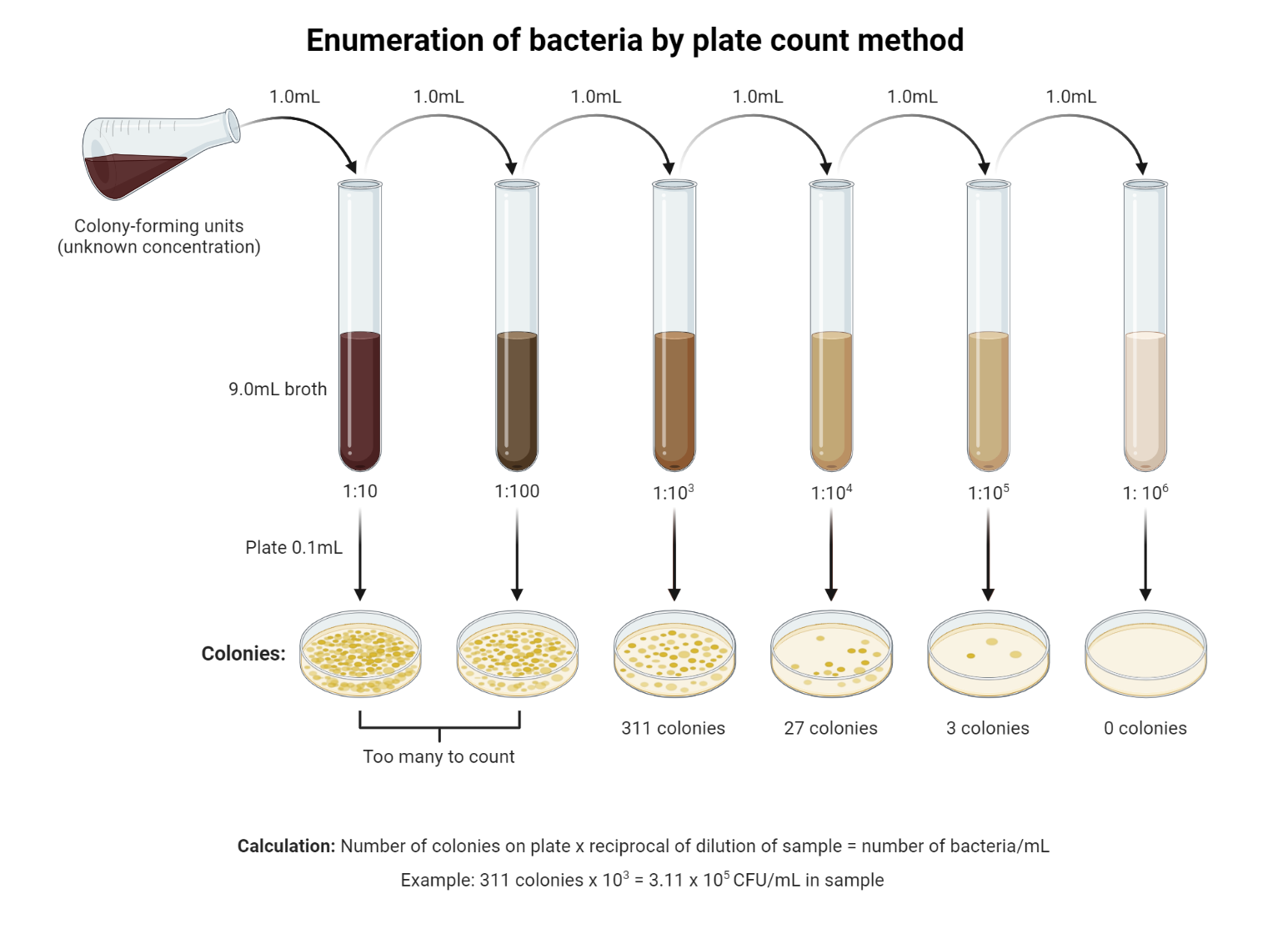
- Write 10-1, 10-2, 10-3, 10-4, 10-5, 10-6, and 10-7 in the dilution boxes.
- Prepare the first dilution by putting 1 ml or 1 g of the sample into a 9 ml dilution blank marked 10-1. This dilutes the original sample 10 times
- To make sure the organisms are spread out evenly, mix them well (cells).
- From the first dilution, move 1 ml of the suspension while in motion to the dilution blank 10-2 with a clean and sterile 1 ml pipette. This will dilute the original specimen/suspension 100 times (1/100 or 10-2).
- With a new, clean pipette, move 1 ml of the 10-2 suspension to the 10-3 dilution blank. This makes the original sample 1000 times weaker (10-3).
- Use a new, clean, sterile pipette each time until the original sample has been diluted 10–7 times.
- With the right pipettes, add 1ml or 0.1ml of suspension from the right dilutions (10-1 to 10-7) to sterile Petri dishes while moving. For each dilution, you need to use 2 or 3 Petri dishes.
- Melt the PCA medium and let it cool to 45°C. Add about 15 ml of the melted medium to each Petri plate with the diluted sample. Turn each plate gently to mix the contents and spread the cells throughout the medium.
- Let the plates settle down.
- At 37°C, incubate these plates upside down for 24 to 48 hours.
Results and interpretation
Examine each colony on a plate using a mechanical hand counter and a Quebec colony counter. Only bacterial cell dilutions that have the following properties yield statistically valid plate counts:
Select the plates that have between 30 and 300 colonies produced. Plates with more than 300 colonies are too numerous to count (TNTC) because it is impossible to count them and plates with less than 30 colonies are too few to count (TFTC). Only count plates with between 30 and 300 colonies on them. Don’t forget to count both surface and subsurface colonies.
Multiplying the number of colonies counted by the dilution factor yields the number of organisms per milliliter of the original culture:
The number of cells per ml = the number of colonies/dilution factor.
Example:
- Colonies per plate = 50 Dilution factor = 1∶1 x106 (1∶1,000,000) Volume of dilution added to plate = 1 ml 50 x1,000,000 = 50,000,000 or (5 x 107 ) CFUs/ml (colony-forming units)
- Colonies per plate = 50 Dilution factor = 1∶1 x 105 (1∶1,00,000) Volume of dilution added to plate = 0.1 ml 50 x 100,000 = 50,000,000 (5 x 106 ) cells/0.1 ml 5,000,000 x10 = 50,000,000 (5 x 107 ) CFUs/ml
- In the lab report, note your findings and the computed bacterial counts per ml of material.
- Because the dilutions that were plated were duplicates of one another, calculate the average of the duplicate bacterial counts per milliliter of material and record that information in the lab report’s chart.
Enlist the precautions of the Plate count Method.
- Observe the correct safety procedures. Consider every unidentified or clinical specimen dangerous and adhere to safety procedures accordingly.
- All Petri plates and mediums must be sterilized before use. Sterile conditions must prevail in the workplace.
- It is necessary to utilize sterile water or other media to serially dilute or dissolve the solid sample.
- The solvent that is used to dissolve the solid sample must be sterile and must not have any impact on microorganism growth or activity.
- It is necessary to dilute the sample to a level where the viable microbial load ranges from 20 to 300 CFU/mL. It will be challenging to count the colonies above this range, and the colonies might merge. The result is reported as insignificant below this range. If the colony count is lower than 20 CFU/mL or more than 300 CFU/mL, the procedure must be repeated under the same circumstances.
- When preparing material for serial dilution, precise measurement is essential.
- Transferring samples into a Petri plate, medium tube, or bottle requires the use of a micropipette or a standard calibrated tube.
- The US FDA only allows 12 to 15 mL of medium per 10 cm Petri plate, whereas the USP allows 15 to 20 mL per 10 cm Petri plate.
- Before the agar solidifies, the sample must be well combined with the molten substance.
- The media’s temperature when being poured or mixed with the sample is crucial. It must range from 40 to 45 C. (55 C is the maximum temperature and must not be more than this at any cost). It is not advised to utilize media at temperatures below 400C since they may start to clump or solidify.
- Before adding any media or samples, each Petri plate needs to be labeled. Labeling regarding the dilution factor is required. The labeling must be examined during sample dispensing, and the information regarding the dilution factor must match the concentration of the sample.
Write the applications of the Plate count Method.
- Scientists use it to calculate the concentration of cells in a given sample and to obtain bacterial growth curves.
- It is also used to examine the impact of various environmental and growth factors on the rate of bacterial growth.
What are the advantages and disadvantages of the Plate count Method?
Advantages:
- Counting viable colonies is helpful.
- It is also capable of detecting extremely low bacterial numbers.
- Agar plates that have already solidified are not necessary.
- Additionally, it can be applied to environmental and clinical materials.
Disadvantages:
- Compared to the streak plate/and/or spread plate approach, the Plate count method requires more time to prepare.
- Heat-sensitive organisms that come into contact with heated agar lose their viability. The organism being counted must be able to endure a brief exposure to molten agar’s temperature (between 45°C and 50°C).
- Compared to colonies that are on the surface, embedded colonies are substantially smaller. As a result, it is important to count them carefully to ensure that none are missed.
- The slower rate of growth of obligatory aerobes in the agar’s depth.
References:
https://www.onlinebiologynotes.com/enumeration-of-bacteria-by-plate-count-technique/
https://microbenotes.com/pour-plate-technique-procedure-significance-advantages-limitations/