In nature, microbial populations are not separated by species but exist with a mixture of many different types of cells. In the laboratory, these populations can be separated to make pure cultures by different culturing techniques. These cultures only have one kind of organism and are good for studying their cultural, morphological, and biochemical properties.
Colonies are defined as groups of growing microorganisms on a solid surface that can be seen with the naked eye. Each colony is made up of the offspring of a single organism. Once you have these separate colonies, you will put them on nutrient agar slants in a clean way to separate pure cultures.
Most of the methods for isolating separate colonies require that the number of organisms in the inoculum be decreased first. The smaller population size ensures that after inoculation, the cells are far enough apart to tell the different species apart on the surface of the agar medium.
Methods for culturing technique
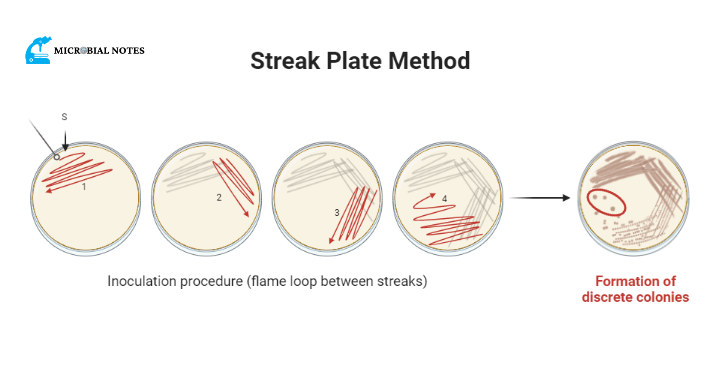
Streak plate method
The streak-plate method is the most common way for microbiologists to get pure cultures. It is easy, simple, and uses as few materials as possible. But you need a certain level of skill that you can only get through practice.
It is important to leave enough space between the colonies on the plate so that a single, pure colony can be taken from quadrant 4 and used for further testing and study. This will make sure that you aren’t working with a culture media that has been mixed or tainted.
All of the methods involve spreading cells out over the surface of a plate until a single cell settles in one spot and grows into an isolated bacterial colony.
Types of the streak plate method
Quadrant streak plate method
Start with the “s” point marked on the figure and move one loop of organisms back and forth over Area 1.
- Use the loop gently.
- Don’t make holes in the culturing medium.
- Make the loop red hot, let it cool for 5 seconds, and then touch the culturing medium briefly in a clean area to make sure it is cool.
- Turn the dish 90 degrees while keeping the lid closed. Streak Area 2 with several back-and-forth strokes, hitting the first streak a few times.
- Make the loop red hot again. Turn the dish and streak through Area 3, making sure to hit the last area more than once.
- Burn the loop, let it cool, and turn the dish another 90 degrees.
- Streak Area 4, get in touch with Area 3 more than once and pull out the culture as shown.
- Make the loop red hot before putting it down.
Radiant streak method
- In Area 1, put a loop’s worth of organisms in a small area near the edge of the plate. Use the loop with care. Don’t make holes in the culturing medium.
- Burn the loop on fire and wait 5 seconds for it to cool down. If you touch a clean area, you will stay cool.
- Make 7 or 8 straight lines from the edge of Area 1 to the other side of the plate.
- burn the loop again, let it cool down enough, and start near Area 1 to cross the streak over the last streaks.
- burn the loop on fire before you put it in its place.
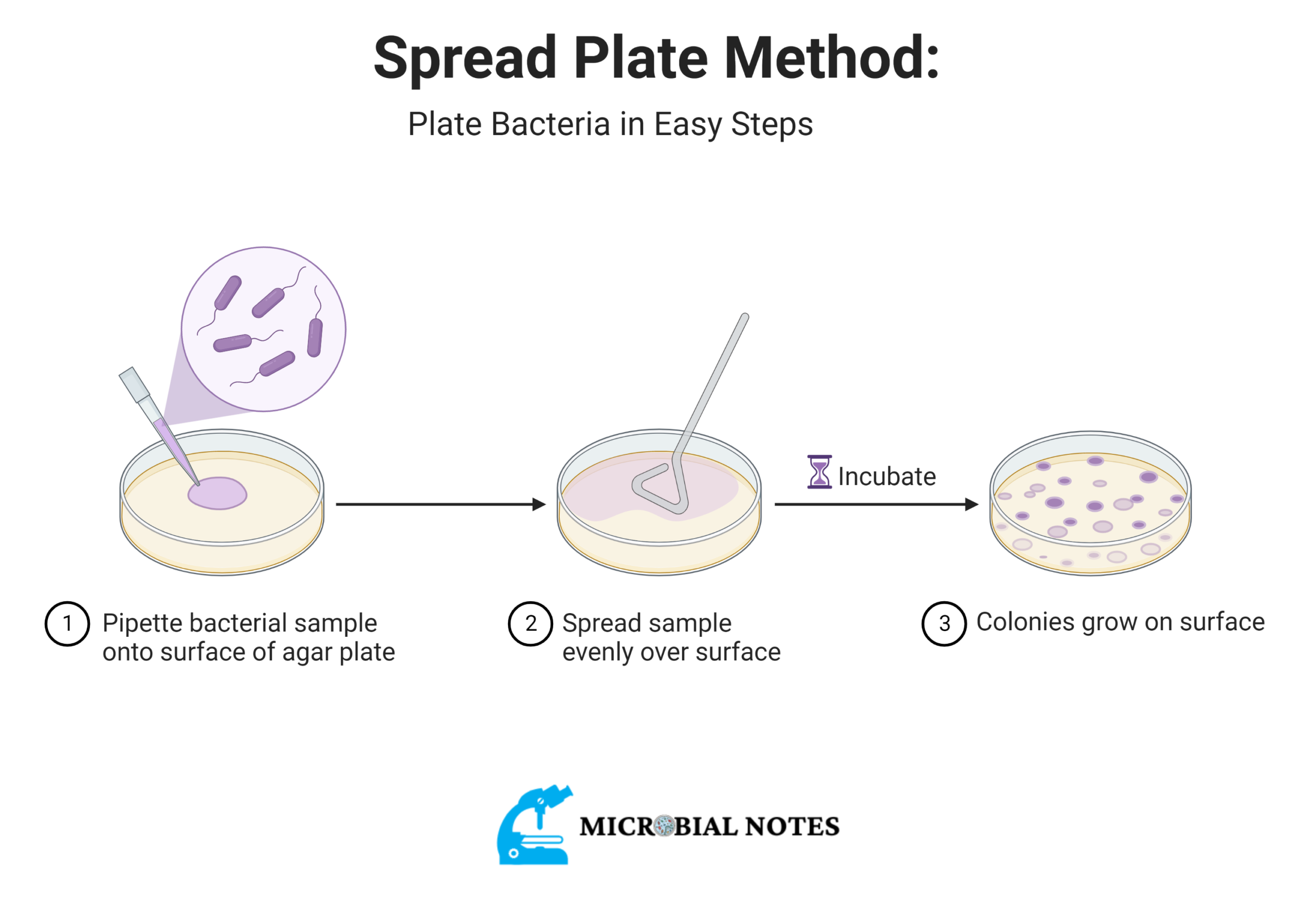
Spread plate technique
For the spread-plate method, you must use a mixture of microorganisms that has already been diluted. During inoculation, the cells are spread over a solid agar medium with a sterile L-shaped bent glass rod while the Petri dish is turned on a “lazy Susan” turntable.
Procedure
- Put the bent glass rod into a beaker and add enough 95% ethyl alcohol to cover the bent part at the bottom.
- Put a labeled nutrient agar plate on the turntable. Using a sterile pipette, put one drop of sterile water in the middle of the plate. Then, put one loopful of sterile bacteria ( e.g Micrococcus luteus ) on top of the water drop. Mix slowly with the loop, and then put the lid back on.
- Take the glass rod out of the beaker and pass it through the flame of the Bunsen burner so that the burning alcohol doesn’t run down your arm. The bent end of the rod should point down. Let all of the alcohol burn off of the rod. Give the rod 10 to 15 seconds to cool down.
- Take off the lid of the Petri dish and turn the turntable.
- While the turntable is spinning, move the sterile bent rod back and forth over the surface of the agar. This will spread the culture across the surface of the agar.
- When the turntable stops, put the cover back on. Soak the rod in alcohol and burn it again on the burner.
- If you don’t have a turntable, turn the Petri dish by hand and use the clean bent glass rod to spread the culture.
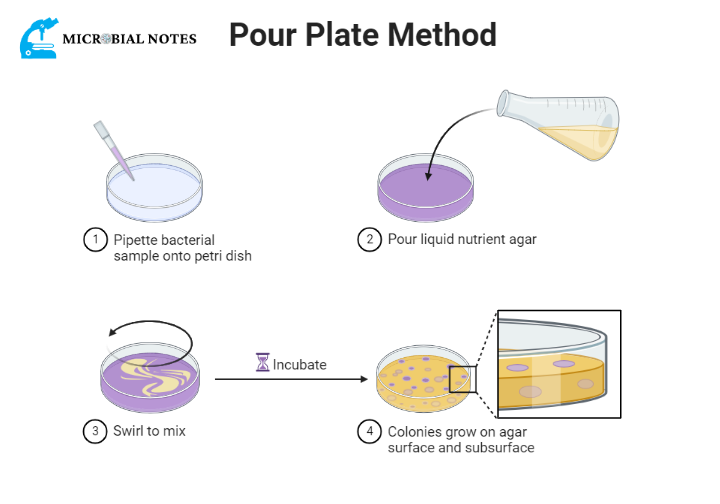
Pour plate method
This method for separating one type of bacteria from another is to mix one loopful of organisms with three tubes of molten nutrient agar in such a way that one of the plates will have the right number of organisms for good isolation.
One advantage of this method is that it doesn’t require as much skill as making a good streak plate culturing technique. On the other hand, it does require more culturing media, tubes, and plates, which is a disadvantage.
also, read standard plate count method its definition technique and results
Procedure
- Make the dilutions of the sample ( which contain bacteria ) according to sample protocol and label the dilutions of each tube.
- Label the Petri dishes according to respective dilutions.
- Innoculate sample dilutions through a pipette in the center of the Petri dishes
- Add molten nutrient agar to each Petri dish
- Swirl the Petri dishes for mixing and let them solidify
- After solidification, invert the microscope and Incubate at 37 C for 24 hours
Resources
Microbiology: A laboratory manual