Introduction
The process of Minimum inhibitory concentration (MIC) testing involves exposing microorganisms to varying concentrations of an antimicrobial agent with the aim of identifying the minimum concentration that inhibits their observable growth. Microbial enumeration is commonly carried out through dilution techniques or gradient diffusion techniques.
The use of dilution techniques, such as broth dilution and agar dilution methods, requires the preparation of serial dilutions of the antibiotics. The microorganism is then introduced into the agar plates or broth.
The use of preformed gradients of antibiotic concentration on agar plates is a common approach in gradient diffusion techniques, such as the Etest and M.I.C Evaluator strip. The minimum inhibitory concentration is determined by quantifying the point of intersection between the growth and the gradient strip.
Factors affecting MIC values:
The susceptibility of microorganisms to antibiotics can be affected by various factors that can alter the Minimum inhibitory concentration values. These include the microorganism characteristics, the environment, and the qualities of the antibiotics.
Characteristics of microorganisms
The inherent properties of microbes can significantly affect their sensitivity to antibiotics, which in turn affects Minimum inhibitory concentration values. The genetic makeup of the bacterium, the presence of particular resistance mechanisms, and the capacity to build biofilms are some of these traits.
Intra-species genetic diversity among microorganisms can lead to varying degrees of susceptibility toward particular antibiotics. Because some microbes have genes that confer resistance to specific antimicrobial drugs, they are less vulnerable to those agents at lower concentrations.
Environmental Factors
The susceptibility of microorganisms to antibiotics can be influenced by the environmental factors, which can affect the minimum inhibitory concentration values. The susceptibility of microorganisms can be influenced by various factors including pH, temperature, oxygen levels, and the presence of other substances.
Changes in pH levels can modify the ionization state of an antibiotic, thereby impacting its potential ability to penetrate the microbial cell membrane and execute its antimicrobial activities. Temperature: Microbial metabolic activity can be influenced by temperature fluctuations, which may have an impact on antibiotic susceptibility.
The MIC values can be influenced by the presence of other substances, such as growth factors or competing microorganisms. Certain substances have the potential to either Increase or reduce the efficacy of antibiotics, thereby causing fluctuations in minimum inhibitory concentration outcomes.
Characteristics of Antibiotics:
The characteristics of the antibiotic itself are very important in determining Minimum inhibitory concentration levels. Antibiotics of different types exhibit distinct modes of entry into microbial cells, chemical structures, and mechanisms of action.
An antibiotic’s mode of action describes the particular procedure or target inside the bacterium. Some antibiotics prevent the formation of cell walls, while others take aim at protein synthesis or DNA replication. The precise mechanism of action can have an effect on the susceptibility of microorganisms to the antimicrobial agent, which can consequently influence the Minimum inhibitory concentration measurements.
The antimicrobial efficacy of an antibiotic is also influenced by its chemical composition, which determines its capacity to invade microbial cells and exhibits antimicrobial properties. Antibiotics with lipophilic properties exhibit higher permeability across cellular membranes than hydrophilic antibiotics, thereby leading to varied Minimum inhibitory concentration values.
Methods for testing MIC.
MIC testing uses diverse techniques to determine the minimum inhibitory concentration of antimicrobial agents against microorganisms.
Methods dilution
The use of dilution methods is common in MIC analysis, where Serial dilutions of the antimicrobial agent are formulated to determine the Minimum inhibitory concentration. The broth and agar dilution methods are two frequently used dilution techniques.
Broth dilution method
The broth dilution method involves the formation of a sequence of tubes or microplates that hold fluid growth media with varying concentrations of the antimicrobial agent. Inoculation of microorganisms is carried out into individual tubes or wells followed by incubation. The minimum inhibitory concentration by broth dilution is defined as the least concentration of the antimicrobial agent that prevents observable growth. This methodology yields quantitative minimum inhibitory concentration values.
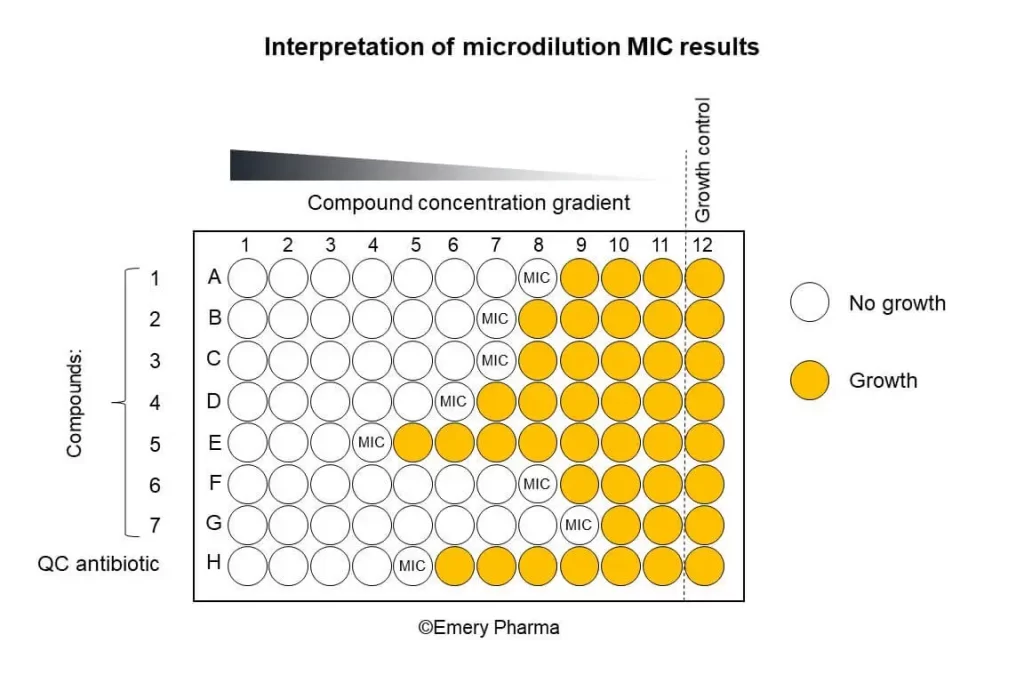
Agar dilution method
The agar dilution method is similar to the broth dilution method but involves the addition of different concentrations of the antimicrobial agent directly into agar plates, Just like in Kirby bauer method, The microbial inoculum is streaked onto the agar plates and subjected to incubation. It is very helpful for testing microorganisms that need solid substrate to grow since it produces measurable Minimum inhibitory concentration values.
Gradient diffusion Method.
The use of preformed gradients of antimicrobial agents on agar plates is a common practice in gradient diffusion methods. This method enables the determination of MIC based on the point of growth inhibition. E test and M.I.C.Evaluator strip are two frequently used gradient diffusion techniques in microbiology.
E-test
The E-test methodology refers to the application of a plastic strip that consists of a pre-determined concentration gradient of the antimicrobial agent on an agar plate that has been previously inoculated with the microorganism. Following incubation, a visible zone of inhibition appears along the strip. The minimum inhibitory concentration (MIC) by E-test is determined by observing the point of intersection between the edge of the elliptical zone of inhibition and the strip, which gives a precise quantitative value for the Minimum inhibitory concentration.
Microbial susceptibility testing strip.
The M.I.C.Evaluator strip uses a gradient diffusion technique by utilizing paper strips that are coated with an antimicrobial agent gradient. The antimicrobial strip is carefully placed on the surface of the agar plate, and, it generates a visible zone of inhibition. The minimum inhibitory concentration (MIC) is determined by a comparative analysis of the strip against a reference chart, thereby providing a quantitative value for the Minimum inhibitory concentration.
Automated methodologies for MIC values.
Automation of MIC determination processes has been made possible by technological advancements. These methodologies offer consistent and efficient Minimum Inhibitory Concentration assessment.
VITEK
The VITEK system is an automated tool that employs fluorometric or colorimetric techniques to quantify minimum inhibitory concentration (MIC) values. The methodology integrates dilution techniques with cutting-edge technology and software algorithms to yield precise and effective MIC determinations. The VITEK systems are a prominent tool utilized in clinical microbiology laboratories.
Interpretation of MIC Values.
In most cases, MIC values are expressed as quantitative values (for example, “MIC = 2 g/mL”) or as qualitative categories (for example, “susceptible, intermediate, or resistant”). The interpretation of the results depends on the predetermined guidelines and breakpoints that are unique to every combination of microorganisms and antimicrobial agents.
Clinical Breakpoints and Susceptibility Categories
Pre-determined MIC values known as clinical breakpoints are used to classify microorganisms as either susceptible, intermediate, or resistant to a specific antimicrobial agent. The determination of breakpoints is carried out by regulatory bodies, including the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
Microorganisms exhibiting MIC values lower than the clinical breakpoint are considered susceptible to the antimicrobial agent. This suggests that the treatment of illnesses brought on by these microorganisms will probably be successful with this antibiotic.
Intermediate: The phrase “intermediate susceptibility” refers to the possibility that the antimicrobial agent may still be active against the bacterium despite the need for higher concentrations or alternate treatment methods. Therapeutic interventions may be subject to variation based on the unique clinical circumstances.
The term “resistant” refers to microorganisms having MIC values higher than the recognized clinical breakpoint. This suggests that treating illnesses brought on by these microorganisms will probably not be successful with antimicrobial medication. It is advisable to consider alternative therapeutic modalities.
Application of MIC
MIC testing is an essential aspect of microbiology that finds application in diverse areas.
Surveillance of antibiotic resistance.
The monitoring of antibiotic resistance is made possible via MIC testing. Through the determination of MIC values against diverse antibiotics, microbiologists and healthcare practitioners can track the increase and transmission of antibiotic resistance among different microorganisms. Monitoring alterations in MIC values over a period of time facilitates the recognition of patterns in resistance and allows for the application of suitable measures for infection control. This data informs the formulation of strategies to address antimicrobial resistance at the regional, national, and worldwide levels.
Antibiotic selection for therapeutic purposes:
MIC testing offers useful information for choosing the best medicines to treat infections. Clinicians can evaluate an organism’s sensitivity to various antimicrobial agents by determining the MIC values of that organism. With the best possible efficacy against the pathogenic bacteria, suitable antibiotics can be chosen with the use of this knowledge.
Investigation and exploration of novel antimicrobial agents.
For the purpose of discovering and creating novel antimicrobial drugs, MIC testing is crucial. In the hope of addressing antibiotic resistance, microbiologists investigate the MIC of newly discovered compounds against a diverse array of microorganisms.
Advancements in MIC
In order to increase the precision, speed, and effectiveness of obtaining MIC values. Need more advancements and technologies.
Rapid methods for MIC determination.
Traditional MIC testing procedures can be time-consuming since they call for lengthy incubation times in order to detect audible growth or inhibition. Recent technological progress has facilitated the creation of prompt techniques for determining MIC. These methodologies use cutting-edge methodologies and technologies that accelerate the determination of Minimum Inhibitory Concentration (MIC) values, enabling prompt outcomes and timely therapeutic interventions.
Automated systems that use fluorescence or turbidity detection are capable of promptly evaluating microbial growth or inhibition in microplates, leading to a significant decrease in the time required for MIC determination. These systems frequently integrate advanced algorithms and software for data analysis and interpretation, optimizing the workflow and improving precision.
Genotypic Methods for Predicting MIC
Alongside phenotypic testing techniques, genotypic methodologies have emerged as advantageous instruments for calculating MIC values. These techniques use the understanding of distinct genetic markers or mutations linked with antimicrobial resistance. Through the identification of these indicators, one can determine the MIC values for specific antimicrobial agents.
Molecular assays and whole-genome sequencing are genotypic techniques that have significant insights into the existence of resistance genes or mutations in microbial genomes. The identification of these genetic indicators facilitates determining MIC values, thereby accelerating the evaluation of antimicrobial susceptibility.
This methodology presents various benefits, such as the capability to identify resistance mechanisms that may not be evident in conventional phenotypic assays and the possibility of conducting high-throughput analysis of multiple resistance genes concurrently. The use of genotypic techniques has the potential for accelerating and enhancing the precision of MIC value prediction.
Combining MIC data with Clinical Decision Support Systems (CDSS)
The combination of MIC information with clinical decision support systems represents a noteworthy progression in MIC analysis. CDSS are software applications that offer healthcare practitioners immediate, evidence-based suggestions to guide their therapeutic choices. Through the integration of MIC data into these systems, microbiologists can provide clinicians with a comprehensive understanding of antimicrobial susceptibility, enabling them to make informed decisions regarding antibiotic therapy.
Conclusion
MIC testing is an essential technique in the field of microbiology and plays a crucial role in determining the susceptibility of microorganisms to antimicrobial agents. Antimicrobial susceptibility testing furnishes crucial insights into the increase or reduction of microorganisms to particular antimicrobial agents, thereby directing the course of treatment and monitoring purposes. In this manuscript, we have investigated diverse facets of minimum inhibitory concentration (MIC) testing, encompassing its denotation and importance, comprehension of MIC values, techniques for testing, construing MIC outcomes, utilization in distinct domains, the correlation between MIC and antimicrobial resistance, the function of MIC in patient care and communal health, progressions in MIC testing, and the amalgamation of MIC statistics with clinical decision support systems.
References
- Tortora, G. J., Funke, B. R., & Case, C. L. (2021). Microbiology: An introduction. Pearson Education Limited.
- Willey, J. M., Sandman, K. M., Wood, D. H., & Prescott, L. M. (2019). Prescott’s microbiology (11th ed.). McGraw Hill.
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA.
- Clinical Microbiology Procedures Handbook, Fourth Edition. (2016). In Clinical Microbiology Procedures Handbook, Fourth Edition. American Society of Microbiology. https://doi.org/10.1128/9781555818814
- Procop, G. W., Church, D. L., & Koneman, E. W. (2020). Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. Jones & Bartlett Learning.