Hemagglutination Inhibition Assay (HI) is an important technique in the research of viruses, especially influenza viruses. This test provides important information on an individual’s immune response to viral infections and it is also useful in vaccine development, epidemiological studies, and evaluating the efficacy of vaccination programs. Let’s take a closer look at the many important aspects of HI tests.
History of Hemagglutination Inhibition (HI) Assay
The roots of HI testing can be traced back to the mid-twentieth century. These agglutination tests were at first developed to research and learn about the behavior of influenza viruses, which can agglutinate red blood cells. The surface glycoproteins of influenza viruses are hemagglutinin (HA) and neuraminidase (NA). Hemagglutinin is essential for the first infection of host cells and is the target of immune system antibodies.
Scientists like as Jonas Salk and Thomas Francis initiated the establishment of HI testing, laying the groundwork for understanding influenza immunity and paving the route for the development of the first influenza vaccine.
Introduction to Hemagglutination Inhibition (HI) Assay
Nucleic acids of various viruses encode surface proteins known as hemagglutinin (HA), e.g. influenza virus, that agglutinate red blood cells (RBC) of various types. The process by which viral hemagglutinins react with red blood cells to generate a lattice of agglutinated cells that settle unevenly in a tube or microtitre well is known as hemagglutination. RBCs that do not agglutinate clump together to form a thick button or bead.
The hemagglutination inhibition (HAI) assay, which we will cover in this blog, is a variant of the HA assay. The HA assay detects and quantify the virus, whereas the HAI assay assesses the presence and concentration of antibodies that can neutralize the virus in serum samples. The HAI assay, in simple terms, assesses the immune system’s ability to block the HA protein of the virus, thereby inhibiting hemagglutination and providing an indication of the amount of protection against the virus.
Objectives of HI test
The following are important objectives of HI test:
- To test a serum sample for the absence or presence of antibodies against a certain virus.
- To determine the antibody titer in a sample.
- To determine the antigenic connection between distinct viral strains.
- To evaluate vaccination effectiveness by assessing antibody response in immunized individuals.
Principle of HI test
Hemagglutination occurs when viruses and red blood cells interact. However, if virus-infected serum is mixed with RBCs and virus, RBCs will not agglutinate. This phenomenon is known as hemagglutination inhibition. The presence of antibodies in the infected individual’s serum that neutralizes the virus causes the hemagglutinin protein to be occupied and unable to bind and agglutinate RBCs (which shows positive result). Hemagglutination will occur if the patient’s serum lacks antibodies against the surface proteins of the test virus because surface molecules are free to hemagglutinate red blood cells (which shows negative result).
Similarly, hemagglutination will not occur until virus-specific antibodies have been precisely diluted. The presence of non-specific inhibitors of viral haemagglutination as well as naturally occurring erythrocyte agglutinins may complicate the HAI test. Sera must be treated before use to avoid false positive or negative results.
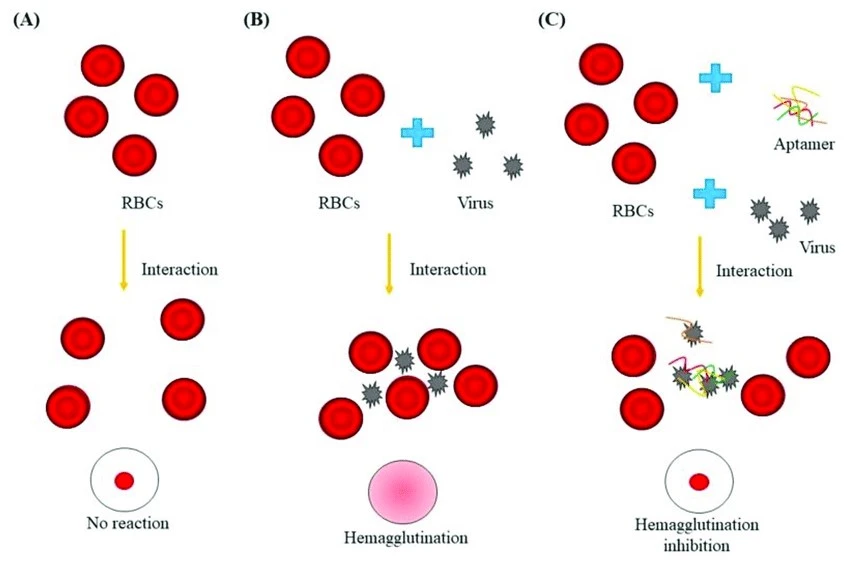
Figure 1: The hemagglutination inhibition assay is shown schematically. (A) RBCs do not bond together and will sink to the well plate’s bottom. This is seen as a red dot in the well’s centre. (B) A number of viruses contain hemagglutinins, which cause RBC agglutination, hemagglutination, and the development of a lattice structure, resulting in a red color throughout the well. (C) The well plate was loaded with virus-specific aptamer, virus, and RBCs. The virus-specific aptamer prevents agglutination, which is apparent as a red dot at the well’s bottom (Kim et al. 2020)
What is HAI titer?
The serum’s HAI titer is the greatest dilution of serum (Ab) that blocks or prevents hemagglutination.
Requirements for Hemagglutination Inhibition (HI) Assay
- Red blood cells from a suitable animal (chicken, guinea pig, goose)
- Diluent at acceptable pH.
- The removal of non-specific hemagglutinin proteins from serum.
- Test tubes or microtiter plates
- Plates for hemagglutination
- The centrifuge
- Micro-pipettes and pipettes
- A standard antigen (e.g., influenza virus) for serology.
Procedure of haemagglutination Inhibition
- Pour diluent or PBS in all twelve wells of 96 well plate.
- Then take the viral sample with a known HA titer (e.g., influenza) or determine its HA titer.
- Prepare 2 folds dilutions of the test serum to be examined, serum dilution will be made up to well 10.
- Then add virus sample up to the 11th well of 96 well plate.
- keep the plate at room temperature for sixty minutes (the period varies depending on your needs).
- Then add RBCs in every well and incubate for 30 minutes at 4 degrees Celsius with red blood cells (RBC).
- After incubation, observe the wells for any bead formation or agglutination.
Result interpretation for HI
The serum’s HAI titer is the greatest dilution of serum (Ab) that suppresses hemagglutination. Agglutination is characterized by a shield of smooth or scattered cells, or by an uneven button. The movement of the red cell button, when the plate is tilted, may help in determining the endpoint.
How to measure haemagglutination inhibition titre?
The reciprocal of the maximum serum dilution that inhibits hemagglutination is the HI titer. The HI titer is 128 if the maximum dilution that prevents hemagglutination is 1:128. A greater titer signifies a greater antibody response to the pathogen.
In the figure below the highest dilution that inhibits haemagglutination is 1:64 so the HAI titre will be 64.
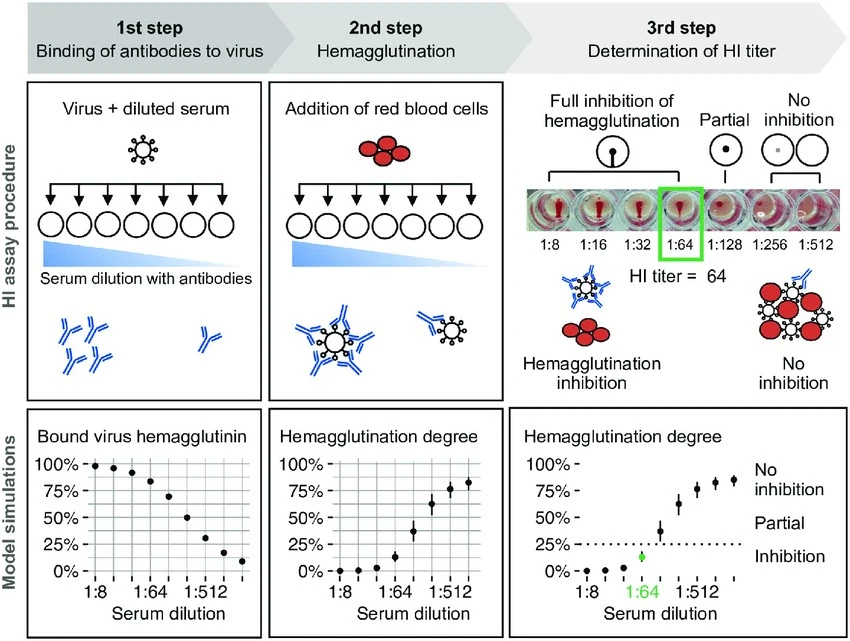
(Linnik et al. 2022).
Applications of hemagglutination inhibition
- The hemagglutination inhibition test is often used to identify infections caused by orthomyxoviruses (influenza), paramyxoviruses (measles, mumps), mononucleosis, arboviruses-togaviruses (including rubella), flavi viruses, and bunyaviruses.
- Measuring antibody responses in vaccinated individuals to assess vaccination efficacy.
- The detection of particular antibodies in a population.
- Hemagglutination can detect the presence of the virus in infected cell cultures; specifically, hemagglutination inhibition can determine the identity of the virus or antibodies in a patient’s serum.
- Antibodies against certain infections can be detected.
- Identifying the antigenic interactions between various viral strains.
- Although the hemagglutination test can detect influenza viruses, the hemagglutination inhibition test is the most effective approach for isol type (HAI).
Advantages of hemagglutination inhibition
- The HA and HI assays are simple assays, use inexpensive and commonly available equipment and supplies, and give results in a matter of hours.
- Specificity and sensitivity are very high.
- Titers of antibodies are quantified.
- Furthermore, the assays are well-established in several laboratories around the world, providing some level of credibility, comparability, and standardization.
- Appropriate for large-scale serological research.
- An established approach with a lengthy history of use.
Disadvantages of hemagglutination inhibition
- Multiple variables, including incubation periods, red blood cell concentration, and red blood cell type, must be optimized for optimal and consistent results.
- Nonspecific components in the sample could produce interference and error in the titer levels. For example, substances in the sample other than viral-specific antibodies can inhibit virus-RBC agglutination and antibody binding to virus.
- False positives can occur due to cross-reactivity with closely similar viruses.
- To avoid non-specific inhibition, materials are typically treated with receptor-destroying enzymes (RDE) prior to examination.
- A trained individual must require to read the plate and determine the titer values in order to analyze the HA or HI data.
- It is both time-consuming and labor-intensive.
- Specific reagents and equipment are required.
- Because of the subjectivity of results and the inconsistent verification of results among human readers, manual interpretation increases the chance of test gaps.
- Furthermore, there is no digital record of the plate or titer measurements, making the first interpretation time-consuming and usually repeated in duplicate. The variety of potential variables and differences between skilled readers may make comparing laboratory data difficult.
Quality control of hemagglutination inhibition
Regular quality control techniques are required to ensure the accuracy of HI results.
- Using positive and negative controls in each test is one of them.
- Equipment should be calibrated on a regular basis.
- Maintaining an assay standard operating procedure (SOP).
- Training employees to accurately execute the assay.
- Validating the assay’s sensitivity and specificity on a regular basis.
Precautions for hemagglutination inhibition assay
Take the following precautions when doing HI:
- In a biosafety level (BSL) laboratory, handle infectious agents.
- Wear the proper personal protection equipment.
- All safety rules and requirements must be followed.
- Handle sharp instruments with caution.
- Properly dispose of hazardous garbage.
Conclusion
Hemagglutination Inhibition is a versatile and valuable technique that can be used in a variety of scientific and medicinal applications. Its ability to assess antibody responses, quantify titers, and detect viruses is critical for improving disease understanding and vaccine development. Researchers can use HI efficiently in their work by following the ideas, materials, and precautions outlined in this blog, leading to the advancement of both medicine and science.
Hemagglutination Inhibition (HI) tests are essential in virology and immunology. They have a long history and help us understand the immunological response to viruses, particularly influenza. These tests influenced vaccine development, public health measures, and provided vital insights into the dynamics of viral infections. While these tests pose challenges, their importance in the area cannot be understated. HI testing will remain an important tool in our fight against infectious illnesses as technology progresses and new problems emerge.
References
- J Katz, PhD, K Hancock, PhD, V Veguilla, MPH, W Zhong, PhD, XH Lu, MD, H Sun, MD, E Butler, MPH, L Dong, MD, PhD, F Liu, MD, PhD, ZN Li, MD, PhD, J DeVos, MPH, P Gargiullo, PhD, N Cox, PhD (2009). Serum Cross-Reactive Antibody Response to a Novel Influenza A (H1N1) Virus After Vaccination with Seasonal Influenza Vaccine Morbid. Mortal. Weekly Rep., 58 (19), 521-524
- Potter, CW, & Oxford, JS (1979). Determinants of immunity to influenza infection in man. Br Med Bull, 35, 69-75
- http://www.virology.ws/2009/05/27/influenza-hemagglutination-inhibition-assay/
Kim S-H, Choi J-W, Kim A-R, Lee S-C, Yoon M-YJB. 2020. Development of ssDNA aptamers for diagnosis and inhibition of the highly pathogenic avian influenza virus subtype H5N1. 10(8): 1116.