Fungi are eukaryotic organisms that are classified into two broad groups: yeast and molds. Their cell walls include chitin. They have a minuscule structure, which is why we can’t see them with our own eyes. By staining them with the appropriate dyes, we can see them under a microscope.
Lactophenol Cotton Blue can be used to stain fungal cells. It is a wet mounting media and the staining solution used to prepare microscopic slides of fungus for study. Fungal components are brightly blue colored.
LPCB ingredients
The most popular technique for coloring and examining fungus is the lactophenol cotton blue (LPCB) wet mount preparation, and it is simple to make. The following ingredients are included in the formulation:
- Phenol kills fungi.
- Lactic acid is a cleaning solution that helps to keep fungal structures intact.
- An aniline dye called cotton blue stains the chitin in the cell walls of fungi, giving the fungus preparation color and highlighting and contrasting the structures.
- Glycerol, a sticky component, keeps the prepared slide specimen from drying out.
Slides are prepared for microscopic fungal examination using lactophenol cotton blue solution as a mounting medium and staining agent. Fungal components are brightly colored blue. Always wear gloves and carry a biological safety hood when performing this treatment.
Principle of LPCB
Lactophenol cotton blue is used in medical mycology to examine the structure of fungal cells by making a wet mount. It is made up of two main components: methyl blue and lactophenol.
Methyl blue is a histological stain that stains collagen blue in tissue slices, whereas lactophenol is a water-based mixture of four components, including phenol, lactic acid, and glycerol. Whereas phenol aids in the killing of germs, lactic acid protects fungal structures and glycerol inhibits the fungus’s cellulolytic action.
When preparing the microscopic slide, methyl blue creates a vivid cerulean color by staining the chitin in the fungal cell walls, and lactophenol functions as a mount.
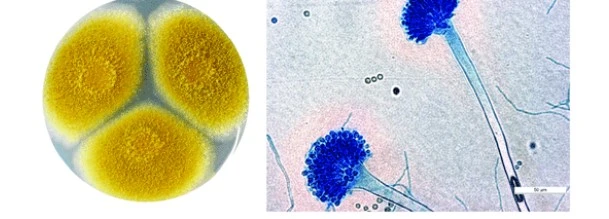
Preparation of LPCB
Lactophenol blue stain takes two days to prepare. Cotton Blue dissolution, phenol crystal dissolution, glycerol addition, filtration, and storage are all crucial phases in the synthesis of LCB.
- Cotton Blue dissolution: For proper dissolution, mix the Cotton Blue with distilled water on the first day and leave it overnight ((this will help to eliminate insoluble dye)).
- Dissolution of Phenol Crystals: On the second day, dissolve phenol crystals in lactic acid using a magnetic stirrer until the phenol is dissolved (Follow proper safety instructions during lactic acid and phenol crystal dissolution).
- Glycerol Addition: Ensure that glycerol dissolves adequately in this mixture of phenol and lactic acid.
- Filtration: Pour the Cotton Blue and distilled water mixer solution into the phenol, glycerol, and lactic acid solution.
- Storage: After preparing the mixer, keep it at room temperature and away from light for subsequent usage. It has a 52-week shelf life from the date of production under these conditions. After purchasing, Lactophenol Cotton Blue should be stored in its original container at 15-25°C in cool, dark and dry place. Under these conditions, the product will be stable until the expiry date listed on the product label.
| Ingredients | Quantity |
| Lactic acid | 20ml |
| Glycerol | 40ml |
| Phenol concentrate Or Phenol crystals | 20ml 20g |
| Distilled water | 20ml |
| Aqueous solution 1% (analogous to cotton blue) Or Aniline blue | 2ml 0.05g |
Pour 20 mL of lactic acid into a beaker and add 40 mL of glycerol. After it add 20 mL of ultrapure water. Finally add 22g of either crystallized phenol or 22 ml of melted phenol. To the prepared mixture, add 0.05g aniline blue. To dissolve the stain, heat the fluid and stir it thoroughly. Do not boil or exceed 100°C. Mix thoroughly and let the solution to cool. The solution can be stored at room temperature and it can be dispensed using a pipette as per the need.
Preparation of LPCB mount
Tease mount method
- Take a clean, greese free glass slide.
- Using a Pasteur pipette or dropper, place a large drop of LPCB on it.
- Transfer a small amount of culture to the drop.
- Tease the culture (in the case of a mould) thoroughly with teasing needles to ensure a uniform spread.
- Apply a coverslip gently to avoid air bubble entrapment.
- Examine with both low-power (10 X) and high-power (40 X) objectives.
- Examine the morphological features as shown below.
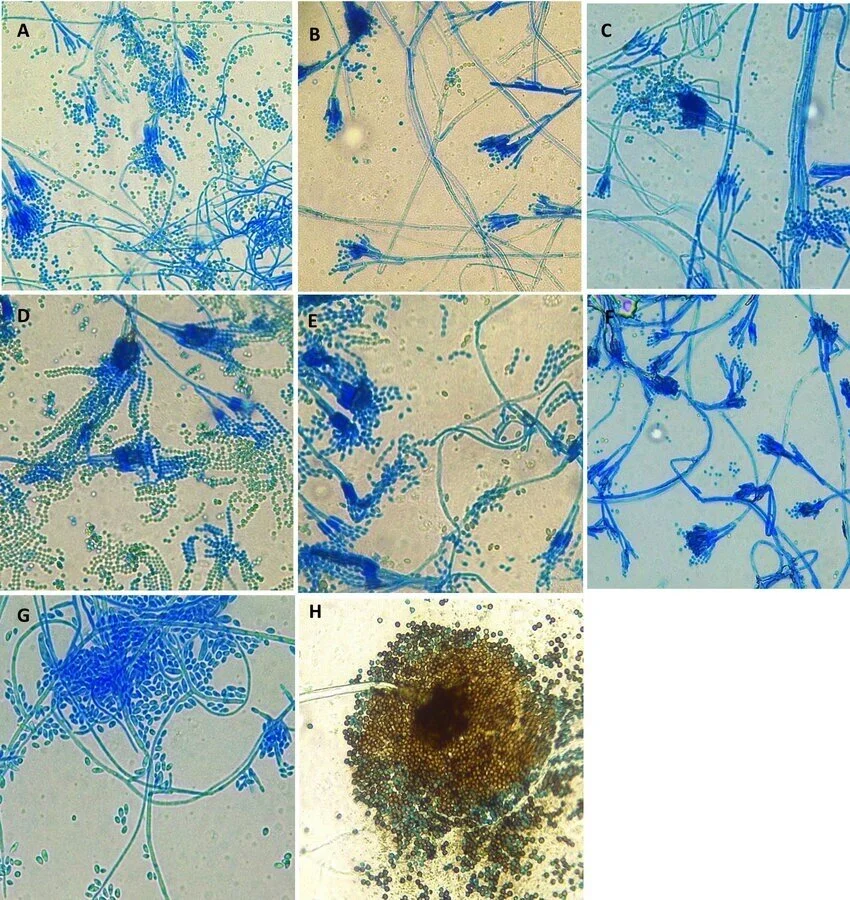
Quality control
- Lactophenol cotton blue solution should be Ink blue in color.
- It should be clear and free of insoluble particles.
- After staining the fungal cell with Lactophenol cotton blue, fungal spores, and hyphae are viewed under a microscope with a high power (40X) objective lens.
- Fungal spores and hyphae will be pale to dark blue in color.
Advantage of LPBS
Lactophenol blue staining helps in the identification of fungal samples as well as its structure.
Disadvantages of LPBS
- Wet mount or Lactophenol cotton blue staining slide cannot be preserved for longer time duration.
- This staining does not allow us to see the fungal cell’s early stages of development.
- It has the potential to affect fungal morphology.
- Lactophenol Cotton Blue is effective for recognizing and presumptively identifying fungus.
- Because the scotch tape mount is just temporary, it will eventually disappear.
- Conidia or spores may be expelled from conidiogenous cells in teasing mount.
Precautions of using LPCB
- Only for in-vitro diagnostic purposes.
- Overheating of organisms during the fixing process may cause organisms on the smear to disintegrate.
- The timing of the decolorizing procedure is determined by the thickness of the smear, and enough allowance should be made based on the tube of the specimen.
- Only properly trained and qualified laboratory professionals should use this product.
References
- GUARDIOLA-MÁRQUEZ, C. E., FIGUEROA-MONTES, M. L., PACHECO MOSCOA, A. & SENÉS-GUERRERO, C. J. S. F. 2021. Native microbial consortia improve maize shoot and root systems at early developmental stages in a seedbed assay. 51.
- HOMA, M., MANIKANDAN, P., SZEKERES, A., KISS, N., KOCSUBÉ, S., KREDICS, L., ALSHEHRI, B., DUKHYIL, A. A. B., REVATHI, R. & NARENDRAN, V. J. F. I. M. 2019. Characterization of Aspergillus tamarii strains from human keratomycoses: molecular identification, antifungal susceptibility patterns and cyclopiazonic acid producing abilities. 10, 2249.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1706009/
What is Lactophenol cotton blue stain?
Lactophenol cotton blue stain is a laboratory technique used to visualize fungi and fungal elements in samples. It is composed of a mixture of lactophenol, cotton blue dye, and other additives that enhance the staining process.
What is the principle of Lactophenol cotton blue stain
The principle behind Lactophenol cotton blue stain is to selectively stain the chitin and cellulose present in the cell walls of fungal elements. Lactophenol is used as a mounting medium, which preserves and clears the sample, while cotton blue dye binds to the chitin and cellulose, producing a blue color that contrasts with the sample background
What are the advantages of using Lactophenol cotton blue stain?
It is a quick and easy staining technique that allows for rapid identification of fungal elements.
The stain produces a clear, distinct blue color that contrasts well with the sample background, making it easy to visualize the fungal elements.
Lactophenol cotton blue stain is versatile and can be applied to a wide range of sample types, including skin, hair, and nails.