Principle of the Semi-Solid agar Method
The semi-solid agar method is a widely used technique in microbiology for determining bacterial motility. The method involves inoculating a bacterial culture into a semi-solid agar medium with a reduced agar concentration (0.2-0.5%) that allows the bacteria to move through the medium by using its organelles such as flagella. The semi-solid agar method is relatively simple, cost-effective, and reliable for assessing bacterial motility in various species.
SIM Medium and its Role in Semi-Solid Agar Method
SIM medium (Sulfide-Indole-Motility medium) is a multipurpose, differential medium commonly used to test for bacterial motility, along with the ability to produce hydrogen sulfide (H2S) and indole from tryptophan.
The SIM medium is a semisolid agar medium that contains three components: iron sulfate, which reacts with hydrogen sulfide to produce a black precipitate; tryptophan, which is used by some microorganisms to produce indole; and a low concentration of agar, which allows the medium to remain semisolid, enabling the detection of bacterial motility.
Additionally, the SIM medium can also detect bacterial motility. If the microorganism is motile, it will move away from the point of inoculation and cause visible turbidity in the semisolid medium. This reaction is used to determine the motility of the microorganism.
Ingredients in SIM medium
The SIM medium is a semisolid agar medium that is composed of several ingredients, including:
Peptone: Peptone is a hydrolyzed protein that is a source of amino acids and peptides that support bacterial growth.
Beef Extract: Beef extract provides essential nutrients, vitamins, and growth factors required for bacterial growth.
Sodium Chloride: Sodium chloride is added to the medium to maintain the osmotic balance of the medium.
Iron Sulfate: Iron sulfate is added to the medium as an indicator for the production of hydrogen sulfide gas. When hydrogen sulfide is produced, it reacts with iron sulfate to form iron sulfide, which is black in color and is visible as a black precipitate.
Tryptophan: Tryptophan is added to the medium as a substrate for the enzyme tryptophanase. Tryptophanase catalyzes the breakdown of tryptophan into indole, pyruvic acid, and ammonia.
Agar: A low concentration of agar is added to the medium to give it a semisolid consistency, which allows the observation of bacterial motility. The semisolid consistency of the medium is also important for the detection of hydrogen sulfide production, as it allows the gas to form a black precipitate in the medium.
Material Required
The following materials are required for the SIM test:
- SIM medium: It is a semisolid agar medium that is composed of several ingredients, including peptone, beef extract, sodium chloride, iron sulfate, tryptophan, and agar. It is important to ensure that the SIM medium is prepared according to the manufacturer’s instructions or laboratory protocols to ensure its accuracy and reliability.
- Inoculation needle: A sterile straight wire inoculation needle or loop is required to pick up a small amount of the bacterial colony from a pure culture or from a mixed culture that has been isolated and purified.
- Bacterial culture: A bacterial culture is required to inoculate the SIM medium. The bacterial culture should be in the log phase of growth and should be pure to ensure accurate and reliable results.
- Incubator: An incubator is required to maintain the appropriate temperature and humidity for the growth of the microorganisms being tested. The SIM medium is typically incubated at a temperature between 35 to 37°C for up to 24 to 48 hours.
- Microscope: A microscope is required to examine the inoculated SIM medium for the presence of specific reactions that indicate motility, indole production, and sulfide production.
- Slides: Microscope slides are required to prepare a smear of the inoculated SIM medium for examination under the microscope. A small amount of the inoculated medium is spread onto the surface of a clean, sterile microscope slide and allowed to air dry. The slide is then heat fixed and stained with a suitable staining reagent to make the microorganisms visible under the microscope.
Procedure
Inoculation of the Medium
The first step of the SIM test is to inoculate the SIM medium. A sterile straight wire inoculation needle or loop is used to pick up a small amount of the bacterial colony from a pure culture or from a mixed culture that has been isolated and purified. The inoculation needle or loop is then used to inoculate the SIM medium by gently stabbing it into the medium to about two-thirds of its depth. The needle is then removed from the medium, and the medium is incubated.
Incubation of the Inoculated Medium:
The inoculated SIM medium is then incubated at the appropriate temperature for the specific microorganism being tested. The incubation period can vary depending on the type of microorganism being tested and the specific laboratory protocols being used. In general, the SIM medium is incubated at a temperature between 35 to 37°C for up to 24 to 48 hours.
Observation of Results:
After incubation, the SIM medium is examined for the presence of specific reactions that indicate motility, indole production, and sulfide production. The following observations are made:
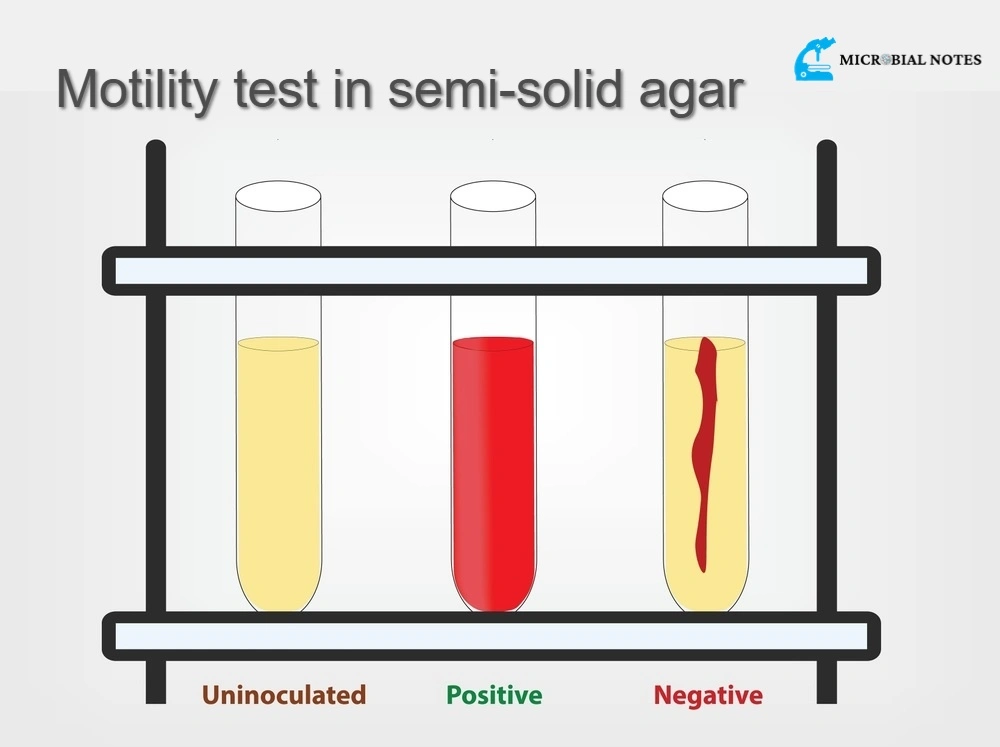
Positive motility test
A positive motility test is indicated by diffuse bacterial growth radiating from the stab line in the semi-solid agar medium. The growth pattern will appear hazy or cloud-like, extending outwards from the original point of inoculation. This indicates that the bacterial species is motile and can move through the medium using structures like flagella or other mechanisms like gliding or twitching motility.
Negative motility test
A negative motility test is characterized by a well-defined, confined growth pattern along the stab line in the semi-solid agar medium. The bacterial growth does not spread out from the initial inoculation point, indicating that the bacterial species is non-motile and lacks the ability to move through the medium.
Troubleshooting and potential errors:
Several factors can affect the accuracy of the motility test results. Some common issues and potential solutions include:
Inappropriate medium consistency: If the agar concentration is too high or too low, it can impact bacterial motility. Ensure the agar concentration is within the recommended range (0.2-0.5%) for semi-solid media.
Inaccurate incubation conditions: Incubation at inappropriate conditions can inhibit bacterial growth and motility, leading to false-negative results. Consult the specific growth requirements of the bacterial species to establish the appropriate incubation conditions.
Over-inoculation: Inoculating with too much bacterial culture can make it difficult to interpret the results, as the growth may be too dense. Use a light, even inoculation to ensure an accurate interpretation of motility patterns.
Contamination: Contamination with other bacterial species can interfere with the test results. Always use aseptic techniques when handling bacterial cultures and preparing the semi-solid agar medium.
Insufficient incubation time: Some bacterial species may require longer incubation periods to exhibit motility. If the results are inconclusive after the initial incubation period, consider extending the incubation time and re-assessing the motility patterns.
Conclusion
The motility test by using SIM medium is a biochemical test used in microbiology to identify and characterize microorganisms based on their ability to produce sulfide, indole, and their motility. The test is relatively simple and inexpensive, making it a widely used tool in clinical diagnosis, research, and quality control.
References
- https://asm.org/ASM/media/Protocol-Images/Motility-Test-Medium-Protocol.pdf?ext=.pdf
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA.
- Tortora, G. J., Funke, B. R., & Case, C. L. (2021). Microbiology: An introduction. Pearson Education Limited.
- Willey, J. M., Sandman, K. M., Wood, D. H., & Prescott, L. M. (2019). Prescott’s microbiology (11th ed.). McGraw Hill.
Frequently Asked Questions
Why is it important to determine bacterial motility?
Bacterial motility is a crucial characteristic that helps in the identification and classification of bacterial species. Additionally, motility plays a role in various biological processes such as colonization, pathogenicity, and biofilm formation.
Can the semi-solid agar method be used for all types of bacteria?
The semi-solid agar method is suitable for most bacterial species, including both gram-positive and gram-negative bacteria. However, some bacteria may require specific modifications to the medium composition or incubation conditions to ensure accurate results.
How can I differentiate between motile and non-motile bacteria using the semi-solid agar method?
Motile bacteria will show a diffuse, cloud-like growth pattern radiating from the stab line, while non-motile bacteria will exhibit confined growth along the stab line without spreading through the medium.