Introduction
The casein hydrolysis test is a laboratory technique used in microbiology to determine the ability of microorganisms to break down casein, a protein present in milk. Casein is a major protein component of milk and serves as a rich source of nutrients for microorganisms. By assessing the ability of microorganisms to hydrolyze casein, the test provides valuable information about their proteolytic (protein-breaking) activity.
Principle of Casein hydrolysis test
Brief Explanation of Casein and Its Significance
Casein (formula: C81H125N22O39P ) is a type of phosphoprotein found in milk, accounting for about 80% of its total protein content. It exists in the form of small spherical particles called micelles, which are composed of various casein subtypes, such as alpha (α)-casein, beta (β)-casein, gamma (γ)-casein, and kappa (κ)-casein. These subtypes differ in their amino acid compositions and have unique properties.
Casein plays a vital role in the nutrition of mammalian infants, providing essential amino acids for growth and development. It is a complete protein, meaning it contains all the essential amino acids required by the body. Moreover, casein has excellent emulsifying and foaming properties, making it useful in the food industry for the production of various dairy products like cheese, yogurt, and ice cream.
Mechanism of Casein Hydrolysis
The mechanism of casein hydrolysis involves the action of proteases on the peptide bonds within the casein protein. When the protease enzyme comes into contact with casein, it binds to the protein’s surface through specific recognition sites. The enzyme’s active site then interacts with the peptide bond, positioning it for hydrolysis.
The protease enzyme catalyzes the hydrolysis reaction by adding a water molecule to the peptide bond. This addition of water breaks the bond, resulting in the separation of the amino acids on either side of the bond. The enzyme’s active site facilitates this reaction by providing an optimal environment for the hydrolysis to occur.
As the enzymatic hydrolysis progresses, multiple peptide bonds within the casein protein are cleaved. This leads to the fragmentation of casein into smaller peptides of varying lengths and free amino acids. The resulting peptides and amino acids can be further utilized by microorganisms as a source of carbon and nitrogen for growth and metabolism.
Materials Required for Casein Hydrolysis Test
- Casein agar or skim milk agar (SMA)
- Sterile distilled water or saline solution
- Inoculating loop or needle
- The incubator set at 35-37°C
- pH indicator paper or pH meter
- Sterile petri plates
Methodology of Casein Hydrolysis Test
Preparation of Media and Reagents
Casein Agar/Skim milk agar
- Prepare a casein agar medium or skimmed milk agar by following these steps
- Dissolve the appropriate amount of casein agar powder or skim milk agar medium in distilled water as per the manufacturer’s instructions.
- Heat the mixture while stirring until the agar is completely dissolved.
- Sterilize the medium by autoclaving at the recommended temperature and pressure.
- Allow the medium to cool to approximately 45-50°C before pouring it into sterile Petri dishes.
Reagents
No additional reagents are required for this procedure
Procedure
- Using a sterile inoculating loop or needle, pick a well-isolated colony of the microorganism to be tested from a pure culture.
- Streak the inoculum onto the surface of the casein agar plate in a zigzag pattern, covering a small area.
- Sterilize the inoculating loop or needle by flaming it and allow it to cool.
- Repeat the streaking process by dragging the loop or needle through the previous streaked area, making a second streak.
- Repeat the streaking process for a third time to obtain isolated colonies.
- Incubate the inoculated plate at the appropriate temperature and conditions.
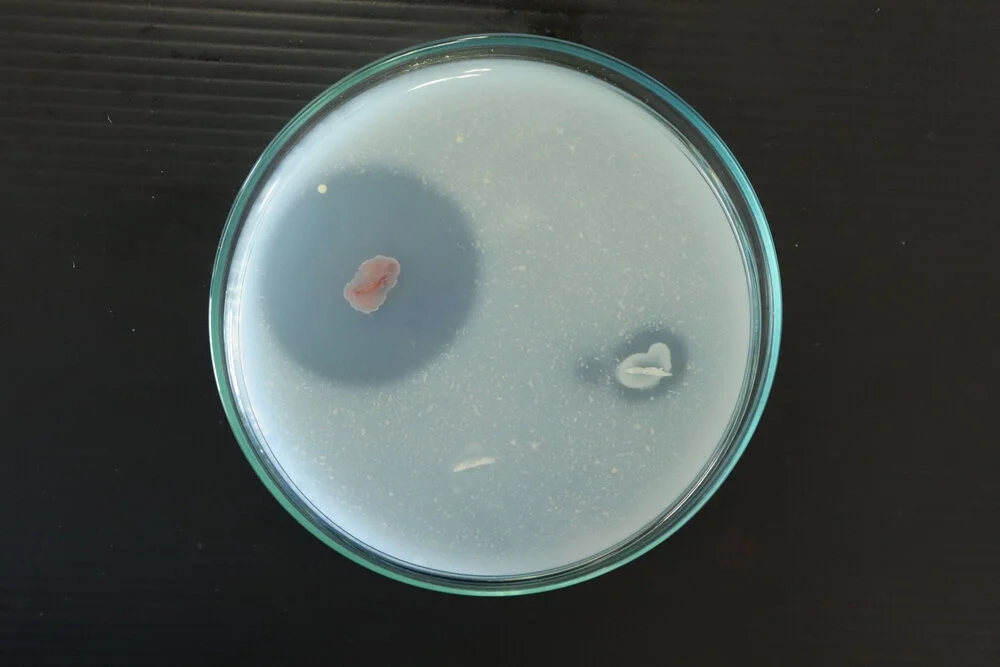
Interpretation of Results
The interpretation of results in the casein hydrolysis test involves observing and analyzing the reactions exhibited by the microbial cultures on the casein agar plates.
- Positive Reaction: A positive reaction is indicated by the presence of a clear zone around the bacterial growth. This clear zone signifies that the casein protein in the agar has been hydrolyzed by the proteolytic enzymes produced by the microorganism. The size of the clear zone can vary depending on the extent of casein hydrolysis.
- Negative Reaction: A negative reaction is observed when no clear zone is present around the bacterial growth. This suggests that the microorganism does not possess the necessary enzymes to hydrolyze casein. In this case, the casein protein remains intact.
Examples of positive and negative results
Some examples of positive and negative results may include
| Positive Casein Hydrolysis | Negative Casein Hydrolysis |
| Bacillus subtilis | Escherichia coli |
| Staphylococcus aureus | Pseudomonas aeruginosa |
| Streptococcus thermophilus | Salmonella enterica |
| Proteus mirabilis | Lactobacillus acidophilus |
| Clostridium perfringens | Enterococcus faecalis |
| Listeria monocytogenes | Acinetobacter baumannii |
| Corynebacterium diphtheriae | Klebsiella pneumoniae |
| Vibrio cholerae | Campylobacter jejuni |
Quality control
To perform any test positve and negative controls must be needed
- For positive control: Pseudomonas aeruginosa ATCC 27853.
- For negative control: E. coli ATCC 29522.
Application of casein hydrolysis test
- Clinical Applications: The casein hydrolysis test has clinical applications in diagnosing infectious diseases caused by proteolytic microorganisms. The test can be applied to various clinical samples, including blood, urine, wound swabs, and respiratory secretions.
- Food industry: The casein hydrolysis test plays a significant role in quality control within the food industry, particularly in dairy product manufacturing. Dairy products such as milk, cheese, and yogurt can be susceptible to spoilage by proteolytic microorganisms. These microorganisms can produce off-flavors, degrade the product’s texture, and reduce its shelf life.
Limitations of casein hydrolysis test
- It needs a long incubation time
- Needs additional biochemical tests for confirmation
References
- Cappuccino J.G. and Sherman N. 2008. Microbiology: A Laboratory Manual, 8th ed. Pearson Benjamin Cummings, San Francisco, CA, USA.
- Clinical Microbiology Procedures Handbook, Fourth Edition. (2016). In Clinical Microbiology Procedures Handbook, Fourth Edition. American Society of Microbiology. https://doi.org/10.1128/9781555818814
- Procop, G. W., Church, D. L., & Koneman, E. W. (2020). Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. Jones & Bartlett Learning.