History of Confocal Microscope
In 1951, while a postdoctoral scholar at Harvard University researching neural networks in the living brain, Marvin Minsky created the confocal microscope (Minsky, 1988). Minsky patented the idea of confocal imaging in 1957, which involves illuminating and detecting a single, diffraction limited region in a specimen. It is a type of light microscope that is using fluorescent and lasers to produce an image The illumination pinhole, specimen plane of focus, and detector pinhole are all located at conjugate focal planes, The technique is known as confocal microscopy.
Confocal microscope definition
One form of 3D Fluorescence Microscope in which resolution is improved by excluding out of focus light is called Confocal Microscope. It is uniquely suited for acquiring 3D images as a collection of intensities at various points in space because of its focusing nature. This is accomplished by employing pinholes with a specified Pinhole Radius, making it an inherent 3D microscope.
The Huygens Confocal Optical Option is ideally suited for Huygens deconvolution on confocal pictures for two key reasons:
1. They continue to exhibit blurring, which is evident when recording bead images.
2. Because the pinhole rejects light from the object, confocal images are typically noisier than widefield images.
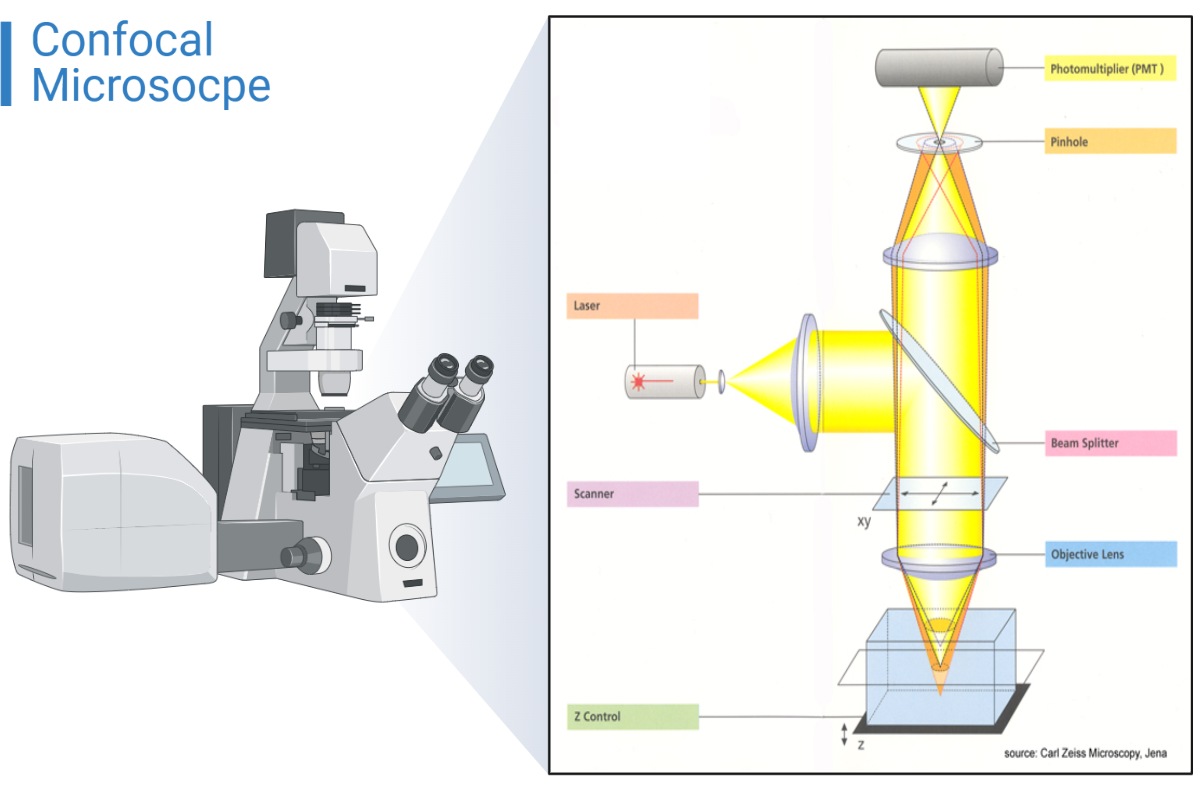
Components of a confocal microscope:
The lessor;
It is compatible with the fluorophores that you will be using in your experiment and can be chosen using a selection device.
Beam splitter;
In the microscope’s fluorescence beam path, it separates the excitation from the light that is released.
Scanner;
It moves a laser beam across the specimen line by line and pixel by pixel.
A fixed lens;
It establishes the system’s resolution and optical image formation.
Z-control;
Because of this, the laser beam can be focused on any focal plane present within the material. We can travel around the axial direction with great precision and small step sizes (about >10 nm) thanks to the motorized Z-stepper.
Pinprick;
A type of changeable iris is a pinhole. Pinholes enable optical sectioning by excluding the majority of out-of-focus light from the captured image. Using the software on the user’s computer, the pinhole’s size can be adjusted.
Photomultiplier tube (PMT);
The photons are changed into an electrical signal, which the computer uses to build an image of the specimen.
Principle of a confocal microscope
The confocal microscope makes use of fluorescence optics, just like the widefield microscope. A defined spot at a particular depth within the sample is illuminated by laser light as opposed to the entire sample being illuminated at once. At this precise moment, fluorescent light is released as a result of this. Only the fluorescence signals from the lighted spot can access the light detector because a pinhole in the optical channel blocks out-of-focus signals.
Images of a single optical plane can be made by raster scanning the specimen. By scanning many optical planes and stacking them with the aid of the right microscopy deconvolution software, 3D objects can be seen (z-stack). Modern confocal microscopes with several lasers and emission/excitation filters can also be used to investigate multicolor immunofluorescence stainings.
How does a confocal microscope work?
The majority of biological systems are three-dimensional structures. The image is produced when the fluorophore-labeled sample is flooded with the excitation of laser light. as traditional wide field microscopy is typically distorted by fluorescent light which is out of focus. This causes the image to become blurry and drastically reduces contrast. A method to cut down on this undesirable out-of-focus light is confocal microscopy. The excitation light is sharply focused inside the sample. The same objective collects the emission light from the focus, which is then focused through a tiny pinhole and sent in the direction of a photodetector. The pinhole will largely block the out-of-focus emission light.
Only the light from the focal volume can enter the detector as a result of this technique. The disadvantage is that while confocal microscopy uses point-wise excitation and point-wise detection, light from other areas of the focus plane is also suppressed. Raster scanning the sample point by point is one way to rebuild a whole image using only the fluorescence signal from the focus plane as the source of data. To cut up thick samples and create a 3D image of the sample, repeat this raster scanning procedure for several focal planes. Alternatively, you might scan the material using numerous excitation sites concurrently, such with a spinning disc microscope.
(Stimulated Emission Depletion Microscopy) STED is essentially a development of confocal point-scanning microscopy. To achieve the high intensity required for stimulated emission, the STED beam must be concentrated in the sample. Point-wise detection lowers the amount of out-of-focus light. The excitation focus center and STED intensity of the doughnut-shaped focus must precisely overlap for STED to function. Raster scanning the sample, for instance using a piezo scanning stage, can then produce an image. The depth of focus of the microscope will depend on the size of the pinhole utilized.
Resolution of a confocal microscope
Confocal microscopes, when used properly, are capable of axial resolutions of 500 nm and lateral resolutions of 180 nm. However, spherical aberration, which can result from mismatched refractive indices, frequently reduces axial resolution in depth.
Applications of a confocal microscope
- It is used to examine different eye conditions.
- used to quantify and analyze the cornea’s endothelial cells on a qualitative level.
- used to identify filamentous fungal components in cases of keratomycosis in the corneal stroma.
- It is also extensively analyzed in the pharmaceutical sector to regulate the consistency and quality of drug distribution.
- used to establish the Magdalen papyrus’ age.
- utilized in systems for 3D optical data storage.
- The recovery of degraded historical audio is another use for it, along with optical scanning.
Advantages and disadvantages of a confocal microscope
Advantages:
- A high-resolution image is produced.
- The specimen is turned into a 3D picture.
- Cells might be living or fixed.
- utilized while collecting serial optical portions.
- The focus spots are evenly illuminated by the confocal microscope.
Disadvantages:
- The cost of confocal microscopes is high.
- It has a small spectrum of excitation wavelengths with very small bands.
Advancements in Confocal Microscopy
Confocal microscopy has undergone significant advancements in recent years, allowing researchers to study microorganisms in unprecedented detail. These advancements have enabled researchers to explore questions that were previously impossible to answer and have expanded the capabilities of confocal microscopy. Some of the key advancements in confocal microscopy are described below:
Super Resolution Confocal Microscopy
Super resolution confocal microscopy is a type of confocal microscopy that enables researchers to resolve structures that are smaller than the diffraction limit of light. This is achieved by using specialized techniques to manipulate the fluorescence emitted by the sample, allowing researchers to obtain images with higher resolution than traditional confocal microscopy. Super resolution confocal microscopy has enabled researchers to study microorganisms in greater detail than ever before, revealing previously unknown structures and enabling researchers to explore new questions about microorganisms.
Multiphoton Excitation Microscopy
Multiphoton excitation microscopy is a type of confocal microscopy that uses infrared light to excite fluorescent molecules within the sample. This technique allows researchers to image deep tissue samples, making it particularly useful for studying microorganisms in complex environments, such as those found in soil or within the human body. Multiphoton excitation microscopy has also enabled researchers to study living organisms over long periods of time, providing new insights into microbial behavior and growth.
Lightsheet Microscopy
Lightsheet microscopy is a type of confocal microscopy that uses a thin sheet of light to illuminate the sample. This allows researchers to rapidly image large samples, making it particularly useful for studying microorganisms in their natural environments. Lightsheet microscopy has enabled researchers to study the behavior of microorganisms in complex communities, such as those found in soil or in the human gut, providing new insights into the interactions between microorganisms and their environment.
Comparison Between Different Types of Advanced Confocal Microscopy
Super resolution confocal microscopy, multiphoton excitation microscopy, and lightsheet microscopy each have their own strengths and weaknesses, making them better suited for certain applications than others. Super resolution confocal microscopy, for example, is ideal for studying the structure of small organelles within cells, while multiphoton excitation microscopy is better suited for studying microorganisms within complex tissues. Lightsheet microscopy, on the other hand, is ideal for studying microorganisms in their natural environments, such as in soil or within the human body.
References:
https://microbiologynote.com/confocal-microscope-principle-uses-parts-advantages-and-disadvantages/